Fenofibrate mitigates the dysfunction of high glucose-driven human retinal microvascular endothelial cells by suppressing NLRP3 inflammasome
- PMID: 40385128
- PMCID: PMC12043296
- DOI: 10.18240/ijo.2025.05.04
Fenofibrate mitigates the dysfunction of high glucose-driven human retinal microvascular endothelial cells by suppressing NLRP3 inflammasome
Abstract
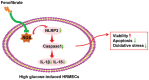
Aim: To determine the therapeutic benefits of fenofibrate (Feno) on the dysfunction of high glucose (HG)-induced human retinal microvascular endothelial cells (HRMECs) and to elucidate the underlying molecular mechanism.
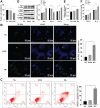
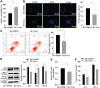
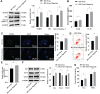
Methods: HRMEC dysfunction model was established by 48h glucose (30 mmol/L) treatment and treated with Feno/NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome activator (Nigericin). Cell viability/apoptosis were assessed by cell counting kit-8 (CCK-8)/terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling assay (TUNEL) staining and flow cytometry assays. Levels of apoptosis- (Bcl-2-associated X protein, Bax/B-cell lymphoma 2, Bcl-2), vascular permeability-(vascular endothelial growth factor, VEGF) and inflammasome activation-related proteins (NLRP3/cleaved caspase-1/apoptosis-associated speck-like protein containing a CARD, ASC), as well as inflammatory factors (interleukin, IL-6/IL-1β/tumor necrosis factor, TNF-α/IL-18) were determined with Western blot/enzyme linked immunosorbent assay (ELISA). Cell permeability/reactive oxygen species (ROS) level/superoxide dismutase (SOD) activity/malondialdehyde (MDA) content were assessed by Evans blue staining/2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescent probe/SOD kit/MDA kit.
Results: HRMEC dysfunction was successfully induced by HG, evidenced by decreased viability (P<0.001), increased apoptosis (P<0.001), permeability (P<0.001), and inflammatory factor levels (P<0.001). Feno treatment significantly ameliorated HG-induced HRMEC dysfunction (P<0.01). Meanwhile, HG induction increased ROS production (P<0.001) and MDA content (P<0.001) in HRMECs, while reducing SOD activity (P<0.001), indicative of oxidative stress. This was, however, abolished by Feno (P<0.05). Moreover, Feno eliminated activation of NLRP3 inflammasomes (P<0.05) in HG-induced HRMECs. Strikingly, activation of NLRP3 inflammasomes partially averted the inhibition of Feno on HG-induced HRMEC dysfunction (P<0.05).
Conclusion: Feno represses oxidative stress and NLRP3 inflammasome activation, consequently alleviating HG-induced HRMEC dysfunction.
Keywords: NOD-like receptor thermal protein domain associated protein 3 inflammasomes; fenofibrate; high glucose; human retinal microvascular endothelial cells; oxidative stress.
International Journal of Ophthalmology Press.
Conflict of interest statement
Conflicts of Interest: Shi Y, None; Chen HM, None; Liu AH, None; Li XR, None.
Figures

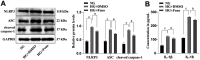
Similar articles
-
Agonism of GPR120 Prevented High Glucose-Induced Apoptosis of Retinal Endothelial Cells through Inhibiting NLRP3 Inflammasome.Klin Monbl Augenheilkd. 2023 Nov;240(11):1292-1299. doi: 10.1055/a-1811-7099. Epub 2022 May 18. Klin Monbl Augenheilkd. 2023. PMID: 35584771 English.
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
Genipin relieves diabetic retinopathy by down-regulation of advanced glycation end products via the mitochondrial metabolism related signaling pathway.World J Diabetes. 2023 Sep 15;14(9):1349-1368. doi: 10.4239/wjd.v14.i9.1349. World J Diabetes. 2023. PMID: 37771331 Free PMC article.
-
[Daidzein attenuates high glucose-induced inflammatory injury in macrophages by regulating NLRP3 inflammasome signaling pathway].Zhongguo Zhong Yao Za Zhi. 2024 Sep;49(17):4734-4743. doi: 10.19540/j.cnki.cjcmm.20240516.706. Zhongguo Zhong Yao Za Zhi. 2024. PMID: 39307808 Chinese.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
