Exploring perspectives of Dscam for cognitive deficits: a review of multifunction for regulating neural wiring in homeostasis
- PMID: 40385132
- PMCID: PMC12081453
- DOI: 10.3389/fnmol.2025.1575348
Exploring perspectives of Dscam for cognitive deficits: a review of multifunction for regulating neural wiring in homeostasis
Abstract
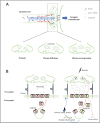
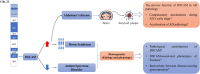
Down syndrome cell adhesion molecule (Dscam) represents a group of cell surface transmembrane receptors with a conserved protein structure across species. In Drosophila, Dscam exhibits extensive isoform diversity resulting from alternative splicing, providing each cell with a unique identity. Identical isoforms expressing on the surfaces of opposing cells mediate homophilic interactions, thereby driving intracellular signaling for establishment of complex neuronal branching patterns. Mammalian Dscam lacks isoform diversity but retains the homophilic binding property. In contrast, it is capable of mediating multifaced neurological functions which are more complex than those of Drosophila Dscam. In this review, we spotlight that the homeostatic mechanisms mediated by Dscam are significant for normal cognitive function. Down syndrome (DS) and autism spectrum disorders (ASD) are two common neurodevelopmental diseases, the cognitive deficits of which are frequently correlated with aberrant DSCAM expression. Previous studies have presented some evidence that the neural homeostatic mechanisms associated with DSCAM are compromised in these two diseases. However, the insight into DSCAM-mediated homeostatic plasticity remains seriously overlooked. Furthermore, recent studies put forward that DSCAM might be one of the key molecules involved in neuronal age-related mechanisms during early stage of Alzheimer's disease (AD), a neurodegenerative disease linked to aberrant homeostatic mechanisms. In this review, we aim to provide a comprehensive understanding of Dscam-mediated crucial roles in regulating neural circuitry for homeostasis, thus elucidating how Dscam induces changes of homeostatic plasticity to affect cognitive function in either physiological or pathological conditions. We hope this review could inspire future studies to test the extent to which Dscam-mediated neural homeostatic mechanisms contribute to neurological disorders accompanied by cognitive deficits, thus facilitating research on discovering potential therapeutic avenues.
Keywords: Alzheimer’s disease; Dscam; cognitive deficits; homeostatic synaptic plasticity; learning and memory.
Copyright © 2025 Xiong, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Dscam diversity is essential for neuronal wiring and self-recognition.Nature. 2007 Sep 13;449(7159):223-7. doi: 10.1038/nature06099. Nature. 2007. PMID: 17851526 Free PMC article.
-
Dscam and DSCAM: complex genes in simple animals, complex animals yet simple genes.Genes Dev. 2009 Jan 15;23(2):147-56. doi: 10.1101/gad.1752909. Genes Dev. 2009. PMID: 19171779 Review.
-
A comparative overview of DSCAM and its multifunctional roles in Drosophila and vertebrates.Neurosci Res. 2024 May;202:1-7. doi: 10.1016/j.neures.2023.12.005. Epub 2023 Dec 21. Neurosci Res. 2024. PMID: 38141781 Review.
-
Got diversity? Wiring the fly brain with Dscam.Trends Biochem Sci. 2006 Oct;31(10):581-8. doi: 10.1016/j.tibs.2006.08.003. Epub 2006 Aug 21. Trends Biochem Sci. 2006. PMID: 16919957 Review.
-
Memory consolidation in honey bees is enhanced by down-regulation of Down syndrome cell adhesion molecule and changes its alternative splicing.Front Mol Neurosci. 2024 Jan 9;16:1322808. doi: 10.3389/fnmol.2023.1322808. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38264345 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

