Surveying Enzyme Crystal Structures Reveals the Commonality of Active-Site Solvent Accessibility and Enzymatic Water Networks
- PMID: 40385134
- PMCID: PMC12079200
- DOI: 10.1021/acsomega.4c10721
Surveying Enzyme Crystal Structures Reveals the Commonality of Active-Site Solvent Accessibility and Enzymatic Water Networks
Abstract
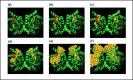
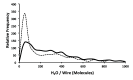
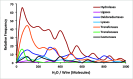
Despite the demonstrable dependence of enzyme functionality on solvation, the notion of water being directly chemically required for catalysis inside active sites remains unexplored. Here we report that over 99% of 1013 enzyme crystals obtained by X-ray crystallography with high resolution (<1.5 Å) contain continuous chains of water linking residues within the active site to bulk water. Also reported are the findings which inspired this study-that electric fields experienced by water hydrogen atoms are on average twice as strong in the active sites of both chains of bacterial polynucleotide kinase (PDB 4QM6) structures compared to those in bulk water. These results point to the possibility that water molecules within active sites may be paramount to the immense catalytic power of enzymes, especially for mechanisms requiring hydronium or hydroxide ions.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Agmon N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244 (5–6), 456–462. 10.1016/0009-2614(95)00905-J. - DOI
LinkOut - more resources
Full Text Sources
