Purine Scaffold in Agents for Cancer Treatment
- PMID: 40385142
- PMCID: PMC12079222
- DOI: 10.1021/acsomega.5c00340
Purine Scaffold in Agents for Cancer Treatment
Abstract
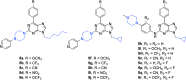
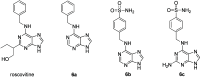
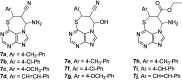
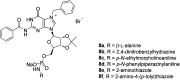
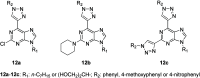
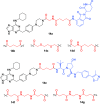
Cancer represents one of the most important and often fatal threats in the human population. Regarding the natural products, the purine scaffold appears in the purine bases in nucleic acids. Purine and its natural derivatives display a number of pharmacological effects. Previous investigations revealed that different compounds bearing the purine scaffold in their molecules belong to a group of potent agents for cancer treatment. Therefore, this review focuses on summarizing recently designed agents for potential cancer treatment bearing the purine scaffold as the key structural motif in the molecules. The reviewed structures clearly show the advantages and disadvantages of different substituents of the key scaffold that affect the final cytotoxic effects of the studied structures. The structure-activity relationship analysis shows a summary of different but potent compounds mentioned in this review and identifies the compounds receiving priority importance due to their high cytotoxicity and exceptional physicochemical characteristics. The effects of metal coordination, the formation of convenient conjugated molecules, and supramolecular self-assembly resulting in the production of biologically active nanovesicles and other nanoassemblies are also demonstrated. The reviewed original studies clearly showed the possible advantages of (a) metal ion coordination, (b) the formation of conjugates, and (c) designing smart and biocompatible nanoassemblies for biological activity in comparison with the characteristics of the parent compounds. This review is based on the most recent articles published in the last two years, 2023-2024, and it represents work with a highly interdisciplinary nature. Even if these original articles are not too numerous within the given period, the investigations published therein have clearly documented the importance of the purine scaffold in pharmacology and in medicinal and supramolecular chemistry.
© 2025 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures
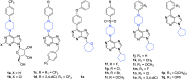
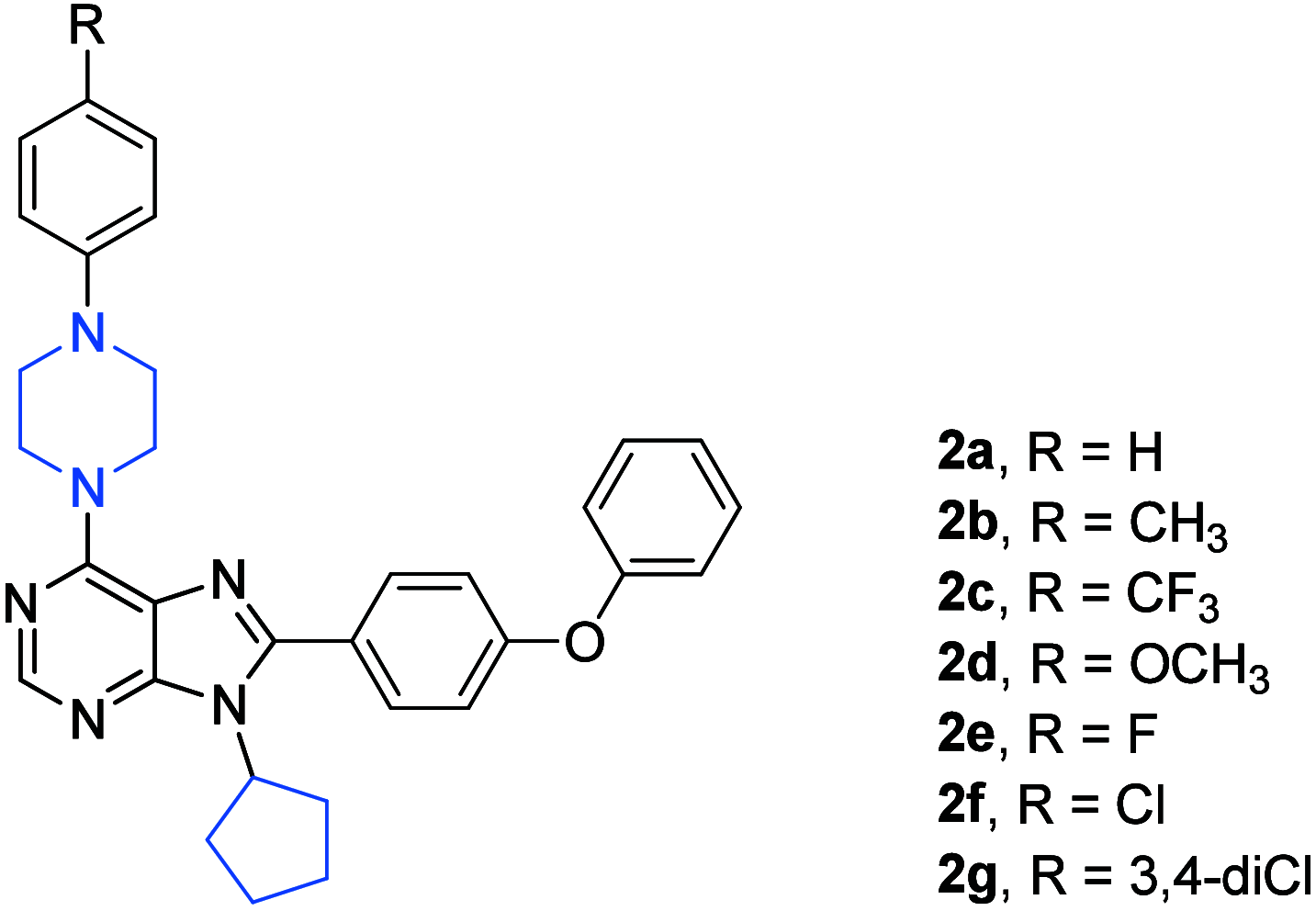
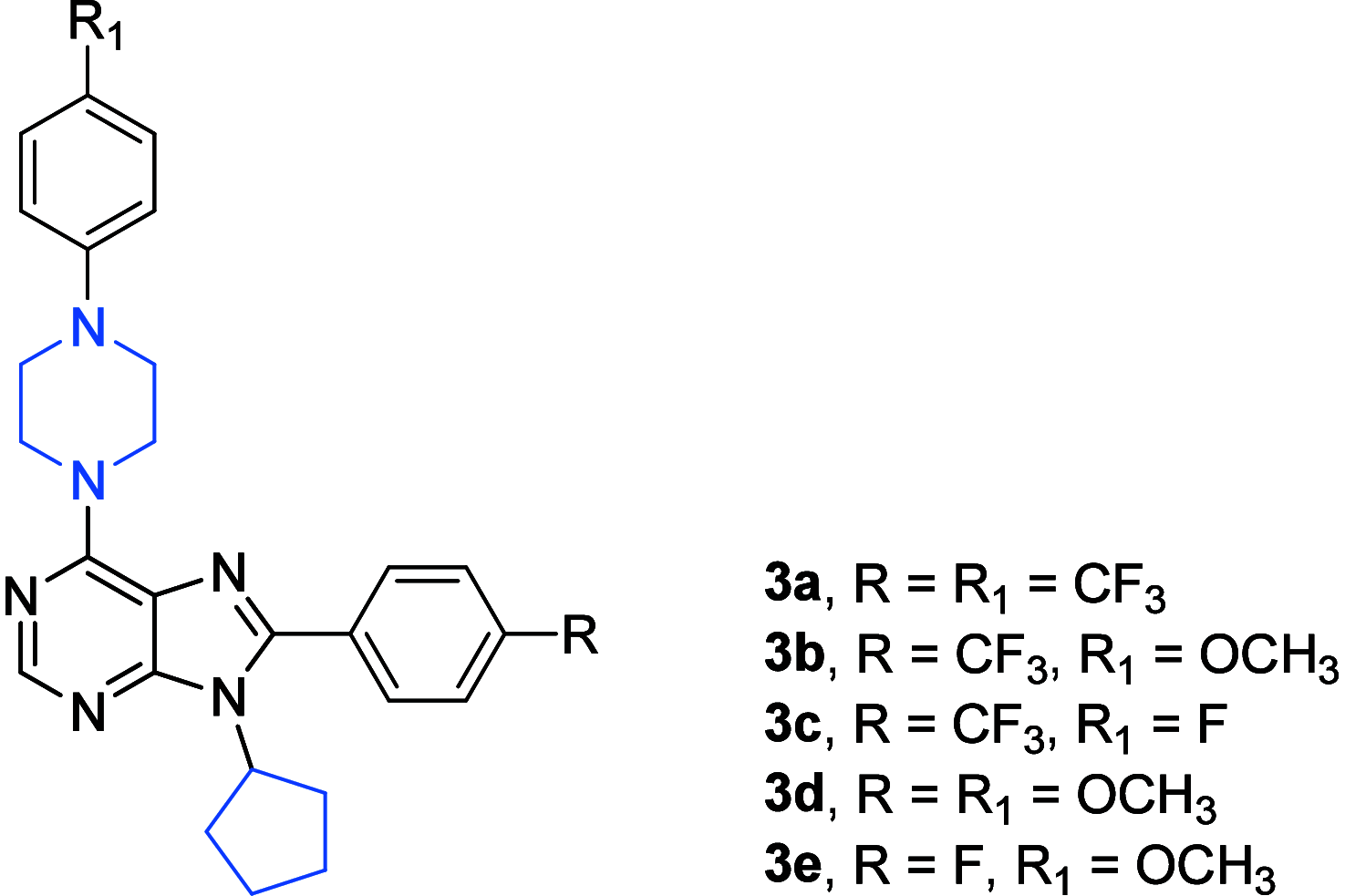
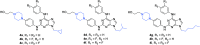

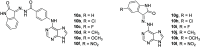
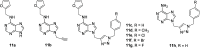
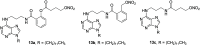
References
-
- Saenger W. In Principles of nucleic acid structure; Springer, New York, NY, 1984.
-
- Das S. K.; Behera B.; Purohit C. S. Scaffolds of purine privilege for biological cytotoxic targets: A review. Pharm. Chem. J. 2023, 57, 777–792. 10.1007/s11094-023-02952-8. - DOI
Publication types
LinkOut - more resources
Full Text Sources
