Low Loading of Collagen in Electrospun Polyester Nerve Conduits for Repairing Segmental Nerve Defect: An Experimental Study Using the Tibial Nerve in Rats with Multiple Measurements
- PMID: 40385186
- PMCID: PMC12079604
- DOI: 10.1021/acsomega.4c10800
Low Loading of Collagen in Electrospun Polyester Nerve Conduits for Repairing Segmental Nerve Defect: An Experimental Study Using the Tibial Nerve in Rats with Multiple Measurements
Abstract
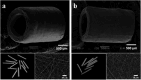
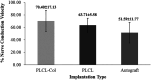
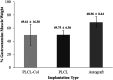
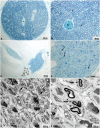
The present study provides in vivo trials of electrospun poly(l-lactide-co-ε-caprolactone), PLCL, copolymer 67:33 mol %, and electrospun PLCL blend with a low loading of collagen (0.5% w/v), PLCL-Col, as a connecting porous biodegradable nerve conduit to repair 7 mm long segmentary tibial nerve lesions in rats compared with the standard autograft technique. The electrospun PLCL scaffolds reveal a matrix of fibers with a mean diameter of 476 ± 60 nm and an average pore size of 253 ± 5 nm. Blending collagen with the PLCL results in a comparatively denser matrix of fibers with a mean diameter of 417 ± 42 nm and a pore size of 244 ± 3 nm. For in vivo testing, a total of 30 male Wistar rats were divided into 3 groups of 10 and each group was subjected to a different nerve repair procedure for evaluation of nerve regeneration after reconstruction. Evaluation of nerve regeneration was compared in terms of the tibial functional index (TFI), nerve conduction velocity (NCV), gastrocnemius muscle weight (%GMW), and a histomorphometric study. After 12 weeks of implantation, there was evidence of nerve regeneration across the gap from the histomorphologic study. All parameters of nerve regeneration were observed in every animal of the study groups. Our results clearly showed that there are reinnervation and return of function in all groups, similarly to the autograft group. PLCL-Col showed better results than PLCL and autograft, which suggested that PLCL-Col porous conduits may serve as a scaffold for peripheral nerve regeneration.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Evaluation of the effectiveness of biodegradable electrospun caprolactoneand poly(lactic acid-ε-caprolactone) nerve conduits for peripheral nerveregenerations in a rat sciatic nerve defect model.Turk J Med Sci. 2016 Feb 17;46(2):539-48. doi: 10.3906/sag-1412-110. Turk J Med Sci. 2016. PMID: 27511522
-
Polymerizing Pyrrole Coated Poly (l-lactic acid-co-ε-caprolactone) (PLCL) Conductive Nanofibrous Conduit Combined with Electric Stimulation for Long-Range Peripheral Nerve Regeneration.Front Mol Neurosci. 2016 Nov 8;9:117. doi: 10.3389/fnmol.2016.00117. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27877111 Free PMC article.
-
Electrospun silk fibroin/poly(lactide-co-ε-caprolactone) nanofibrous scaffolds for bone regeneration.Int J Nanomedicine. 2016 Apr 11;11:1483-500. doi: 10.2147/IJN.S97445. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27114708 Free PMC article.
-
Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing.Acta Biomater. 2022 Mar 15;141:219-232. doi: 10.1016/j.actbio.2022.01.042. Epub 2022 Jan 23. Acta Biomater. 2022. PMID: 35081432
-
Sciatic nerve regeneration in rats by a promising electrospun collagen/poly(ε-caprolactone) nerve conduit with tailored degradation rate.BMC Neurosci. 2011 Jul 15;12:68. doi: 10.1186/1471-2202-12-68. BMC Neurosci. 2011. PMID: 21756368 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
