Design, Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 1,3,4-Thiadiazole Derivatives Targeting Both Aldose Reductase and α-Glucosidase for Diabetes Mellitus
- PMID: 40385215
- PMCID: PMC12079242
- DOI: 10.1021/acsomega.5c00566
Design, Synthesis, Biological Evaluation, and Molecular Docking Studies of Novel 1,3,4-Thiadiazole Derivatives Targeting Both Aldose Reductase and α-Glucosidase for Diabetes Mellitus
Abstract
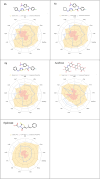
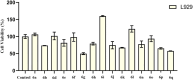
We have developed new 1,3,4-thiadiazole derivatives and examined their ability to inhibit aldose reductase and α-glucosidase. All of the members of the series showed a higher potential of aldose reductase inhibition (K I: 15.39 ± 1.61-176.50 ± 10.69 nM and IC50: 20.16 ± 1.07-175.40 ± 6.97 nM) compared to the reference inhibitor epalrestat (K I: 837.70 ± 53.87 nM, IC50: 265.00 ± 2.26 nM). Furthermore, compounds 6a, 6g, 6h, 6j, 6o, 6p, and 6q showed significantly higher inhibitory activity (K I: 4.48 ± 0.25 μM-15.86 ± 0.92 μM and IC50: 4.68 ± 0.23 μM-34.65 ± 1.78 μM) toward α-glucosidase compared to the reference acarbose (K I: 21.52 ± 2.72 μM, IC50: 132.51 ± 9.86 μM). Molecular docking studies confirmed that the most potent inhibitor of α-GLY, compound 6h (K I : 4.48 ± 0.25 μM), interacts with the target protein 5NN8 through hydrogen bonds as in acarbose. On the other hand, compounds 6o (K I: 15.39 ± 1.61 nM) and 6p (K I: 23.86 ± 2.41 nM), the most potent inhibitors for AR, establish hydrogen bonds with the target protein 4JIR like epalrestat. In silico ADME/T analysis was performed to predict their drug-like properties. A cytotoxicity study was carried out with the L929 fibroblast cell line in vitro, revealing that all of the synthesized compounds were noncytotoxic. Furthermore, AMES test has been added to show the low mutagenic potential of the compounds 6h and 6o.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Synthesis, α-Glucosidase, α-Amylase, and Aldol Reductase Inhibitory Activity with Molecular Docking Study of Novel Imidazo[1,2-a]pyridine Derivatives.ACS Omega. 2024 Oct 11;9(42):42905-42914. doi: 10.1021/acsomega.4c05619. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464438 Free PMC article.
-
Identification of a new class of potent aldose reductase inhibitors: Design, microwave-assisted synthesis, in vitro and in silico evaluation of 2-pyrazolines.Chem Biol Interact. 2021 Aug 25;345:109576. doi: 10.1016/j.cbi.2021.109576. Epub 2021 Jul 9. Chem Biol Interact. 2021. PMID: 34252406
-
In vitro studies of potent aldose reductase inhibitors: Synthesis, characterization, biological evaluation and docking analysis of rhodanine-3-hippuric acid derivatives.Bioorg Chem. 2020 Apr;97:103640. doi: 10.1016/j.bioorg.2020.103640. Epub 2020 Feb 4. Bioorg Chem. 2020. PMID: 32086051
-
Discovery of Hydrazine Clubbed Thiazoles as Potential Antidiabetic Agents: Synthesis, Biological Evaluation, and Molecular Docking Studies.Drug Dev Res. 2025 Feb;86(1):e70060. doi: 10.1002/ddr.70060. Drug Dev Res. 2025. PMID: 39907170 Free PMC article.
-
Novel spiroindoline derivatives targeting aldose reductase against diabetic complications: Bioactivity, cytotoxicity, and molecular modeling studies.Bioorg Chem. 2024 Apr;145:107221. doi: 10.1016/j.bioorg.2024.107221. Epub 2024 Feb 19. Bioorg Chem. 2024. PMID: 38387398
Cited by
-
Computational Profiling of Monoterpenoid Phytochemicals: Insights for Medicinal Chemistry and Drug Design Strategies.Int J Mol Sci. 2025 Aug 8;26(16):7671. doi: 10.3390/ijms26167671. Int J Mol Sci. 2025. PMID: 40868994 Free PMC article.
References
-
- Min X.; Guo S.; Lu Y.; Xu X. Investigation on the inhibition mechanism and binding behavior of cryptolepine to α-glucosidase and its hypoglycemic activity by multi-spectroscopic method. J. Lumin. 2024, 269, 12043710.1016/j.jlumin.2024.120437. - DOI
-
- Wu X. Z.; Zhu W. J.; Lu L.; Hu C. M.; Zheng Y. Y.; Zhang X.; Lin J.; Wu J. Y.; Xiong; Zhang K.; Xu X. T. Synthesis and anti-α-glucosidase activity evaluation of betulinic acid derivatives. Arab. J. Chem. 2023, 16 (5), 10465910.1016/j.arabjc.2023.104659. - DOI
-
- Available online: https://www.statista.com/topics/1723/diabetes/ (accessed Jan 6, 2025).
LinkOut - more resources
Full Text Sources
Research Materials
