Synthesis and Anticancer Activity Evaluation of Novel Carborane-Containing Isoflavonoid Analogues
- PMID: 40385218
- PMCID: PMC12079591
- DOI: 10.1021/acsomega.5c00262
Synthesis and Anticancer Activity Evaluation of Novel Carborane-Containing Isoflavonoid Analogues
Abstract
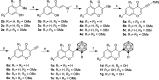
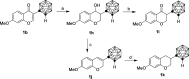
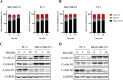
Isoflavonoids represent a privileged structure derived from natural products with diverse bioactivities. Carborane has been utilized as a three-dimensional mimetic of phenyl rings in medicinal chemistry. Herein, we replaced the phenyl group of isoflavonoids with carborane and prepared a series of carborane-containing isoflavonoid analogues. Compounds 1d, 1g, and 1m showed significantly enhanced antiproliferative activities on a broad scope of cancer cell lines. Further studies indicated that both 1d and 1m inhibited JAK/STAT5, PI3K/AKT, and p38 MAPK pathways, leading to G1 cell cycle phase arrest. Additionally, both compounds reduced the expression of P-glycoprotein (P-gp), a key mediator in multidrug resistance, and reversed the resistance of chemotherapeutic agents in multidrug-resistant cells in vitro. The biodistribution of compounds 1d and 1m was evaluated through ICP-mass and positron emission tomography imaging studies. Taken together, these results suggested promising pharmaceutical properties for the carborane-containing isoflavonoid analogues.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Reversing Multidrug Resistance in Chemo-resistant Human Lung Adenocarcinoma (A549/DOX) Cells by Algerian Propolis Through Direct Inhibiting the P-gp Efflux-pump, G0/G1 Cell Cycle Arrest and Apoptosis Induction.Anticancer Agents Med Chem. 2018;18(9):1330-1337. doi: 10.2174/1871520618666180808100800. Anticancer Agents Med Chem. 2018. PMID: 30088453
-
Enhanced reversal of ABCG2-mediated drug resistance by replacing a phenyl ring in baicalein with a meta-carborane.Mol Oncol. 2024 Feb;18(2):280-290. doi: 10.1002/1878-0261.13527. Epub 2023 Oct 5. Mol Oncol. 2024. PMID: 37727134 Free PMC article.
-
Isolation of isoflavones from Iris kashmiriana Baker as potential anti proliferative agents targeting NF-kappaB.Phytochemistry. 2017 Apr;136:70-80. doi: 10.1016/j.phytochem.2017.01.002. Epub 2017 Jan 18. Phytochemistry. 2017. PMID: 28108024
-
Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors.Drug Resist Updat. 2020 May;50:100682. doi: 10.1016/j.drup.2020.100682. Epub 2020 Feb 7. Drug Resist Updat. 2020. PMID: 32087558
-
Carborane-Containing Polymers: Synthesis, Properties, and Applications.ACS Polym Au. 2023 Dec 15;4(1):7-33. doi: 10.1021/acspolymersau.3c00030. eCollection 2024 Feb 14. ACS Polym Au. 2023. PMID: 38371730 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
