Combined use of olfactory mucosal mesenchymal stem cells conditioned medium and neural guide conduits promotes nerve regeneration in an ovine model
- PMID: 40385287
- PMCID: PMC12081432
- DOI: 10.3389/fcell.2025.1598736
Combined use of olfactory mucosal mesenchymal stem cells conditioned medium and neural guide conduits promotes nerve regeneration in an ovine model
Abstract
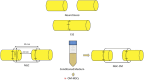
Introduction: Peripheral nerve injuries remain a significant clinical challenge, particularly in severe neurotmesis injuries requiring complex therapeutic interventions to restore functionality. This study aimed to evaluate the pro-regenerative potential of combining neural guide conduits with conditioned medium from olfactory mucosa mesenchymal stem cells, compared to gold-standard surgical techniques.
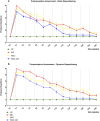
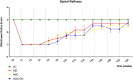
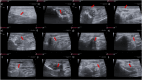
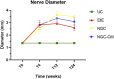
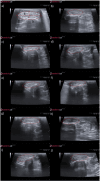
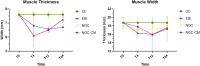
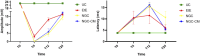
Methods: The study was conducted using a validated ovine model of common peroneal nerve injury. Recovery was assessed over 24 weeks through functional, kinematic, ultrasonographic, and electrophysiological evaluations, complemented by post-mortem nerve stereology and muscle histomorphometry.
Results: All therapeutic approaches promoted nerve and muscle regeneration, resulting in notable functional and structural improvements. However, irregularities were observed, as neural guide conduits and conditioned medium did not consistently outperform standard techniques. Additionally, recovery often fell short of normal values in the control group.
Discussion: These findings highlight the complexity of peripheral nerve regeneration in challenging surgical scenarios and underscore the translational potential of biomaterials and cell conditioned medium-based therapies. However, the observed irregularities emphasize the need for further research in complex animal models before application in real clinical cases. Such studies are essential to refine therapeutic strategies, address inconsistencies, and establish cell conditioned medium as a viable tool in peripheral nerve regeneration and repair.
Keywords: animal model; cell-based therapies; common peroneal nerve; conditioned medium; olfactory mucosa mesenchymal stem cells; peripheral nerve injury; peripheral nerve regeneration; sheep model.
Copyright © 2025 Alvites, Lopes, Coelho, Sousa, Sousa, Moreira, Rêma, Simões, Mendonça, Atayde, Prada, Pires, Silva, João, Metafune, Bertone, Raimondo, Alves, Luís and Maurício.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







Similar articles
-
Effects of Olfactory Mucosa Stem/Stromal Cell and Olfactory Ensheating Cells Secretome on Peripheral Nerve Regeneration.Biomolecules. 2022 Jun 11;12(6):818. doi: 10.3390/biom12060818. Biomolecules. 2022. PMID: 35740943 Free PMC article.
-
Combined Use of Chitosan and Olfactory Mucosa Mesenchymal Stem/Stromal Cells to Promote Peripheral Nerve Regeneration In Vivo.Stem Cells Int. 2021 Jan 2;2021:6613029. doi: 10.1155/2021/6613029. eCollection 2021. Stem Cells Int. 2021. PMID: 33488738 Free PMC article.
-
In vitro evaluation of dental pulp stem cells for sciatic nerve regeneration: foundations for future in vivo applications.Front Cell Dev Biol. 2025 Feb 19;13:1528213. doi: 10.3389/fcell.2025.1528213. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40046233 Free PMC article.
-
Optimizing Peripheral Nerve Regeneration: Surgical Techniques, Biomolecular and Regenerative Strategies-A Narrative Review.Int J Mol Sci. 2025 Apr 20;26(8):3895. doi: 10.3390/ijms26083895. Int J Mol Sci. 2025. PMID: 40332790 Free PMC article. Review.
-
Applications of Proteomics to Nerve Regeneration Research.In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review.
References
-
- Alvites R., Rita Caseiro A., Santos Pedrosa S., Vieira Branquinho M., Ronchi G., Geuna S., et al. (2018a). Peripheral nerve injury and axonotmesis: state of the art and recent advances. Cogent Med. 5 (1), 1466404. 10.1080/2331205x.2018.1466404 - DOI
LinkOut - more resources
Full Text Sources

