Photosynthetic dependence and filament production in physical bacterial-Symbiodiniaceae interactions
- PMID: 40385307
- PMCID: PMC12082827
- DOI: 10.1093/ismeco/ycaf070
Photosynthetic dependence and filament production in physical bacterial-Symbiodiniaceae interactions
Abstract
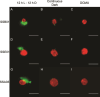
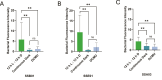
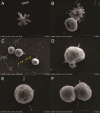
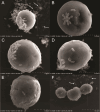
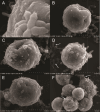
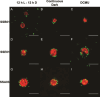
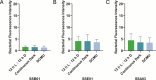
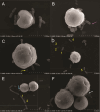
The cnidarian microbiome consists of a wide variety of beneficial microbes that play vital roles in maintaining and fortifying host health. Photosynthesis from symbiotic dinoflagellates (in the family Symbiodiniaceae) is crucial for their symbiosis establishment with the cnidarian host. Although more is known regarding interactions between the host and its associated bacteria and dinoflagellates, there has been little investigation into the relationship between the two microbes themselves and whether photosynthesis plays a role. Through two different methods of photosynthetic inhibition of dinoflagellates (incubation in the dark or pre-treatment with a photosystem II inhibitor), we investigated how pathogenic versus beneficial bacteria physically interact with three Symbiodiniaceae strains (symbiotic and free-living). The beneficial bacterium Tritonibacter mobilis appears to interact with photosynthesizing algae only. In the absence of photosynthesis, little to no physical interactions were observed between Symbiodiniaceae and T. mobilis. Bacterial congregation around individual dinoflagellate cells was significantly lower when photosynthesis was impaired, suggesting photosynthesis is a key facilitator of interactions between T. mobilis and all three Symbiodiniaceae strains. We also investigated whether photosynthesis affects interactions between Symbiodiniaceae and the pathogen Vibrio alginolyticus. Although no discernable impacts of photosynthetic inhibition were observed with the pathogen, scanning electron microscopy uncovered various mechanisms of interaction between Symbiodiniaceae and both bacteria, one of which includes the production of filaments not previously described. Overall, our research highlights the importance of photosynthesis in initiating interactions between bacteria and both free-living and symbiotic dinoflagellates, and opens a door to new questions regarding cell-surface interactions among individual microbes.
Keywords: algae; cnidarian; dinoflagellate; holobiont; microbiome; microscopy; symbiosis.
© The Author(s) 2025. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
None declared.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

