This is a preprint.
Intensity of public health and social measures are associated with effectiveness of SARS-CoV-2 vaccine in test-negative study
- PMID: 40385438
- PMCID: PMC12083626
- DOI: 10.1101/2025.05.08.25327221
Intensity of public health and social measures are associated with effectiveness of SARS-CoV-2 vaccine in test-negative study
Abstract
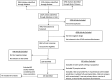
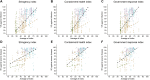
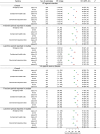
The intensity and duration of exposure can influence vaccine effectiveness (VE). For "leaky" vaccines such as SARS-CoV-2 vaccines, which reduce but do not entirely prevent infections, repeated or prolonged exposures may increase breakthrough infection likelihood. To test this hypothesis, we conducted a systematic review and meta-analysis of 76 test-negative design studies reporting VE against SARS-CoV-2 infection or severe disease. Exposure intensity was approximated using Oxford COVID-19 Government Response Tracker indices: Stringency Index (SI), Containment and Health Index (CHI), and Government Response Index (GRI). Based on 1,419 VE estimates, pooled VE against infection was significantly higher in settings with higher index values (lower exposure intensity): 82% (95% CI: 80-83%) in high-SI settings versus 39% (95% CI: 35-43%) in low-SI settings. Similar patterns appeared for other indices and severe disease outcomes. These associations persisted in meta-regression models adjusting for viral variant, vaccine type, time since vaccination, prior infection status, and enrollment criteria. Correlation analyses showed moderate-to-strong positive correlations between VE estimates and exposure indices (Spearman's correlation: 0.50-0.62). These findings establish exposure intensity as a critical effect modifier of SARS-CoV-2 VE, demonstrating the leaky nature of COVID-19 vaccines and explaining heterogeneity in real-world effectiveness estimates. Future VE evaluations and vaccination strategies should account for exposure intensity to ensure accurate, context-specific estimates.
Keywords: COVID-19; SARS-CoV-2; pre-existing immunity; test-negative design; vaccination; vaccine effectiveness.
Conflict of interest statement
Competing Interests BJC reports honoraria from AstraZeneca, Fosun Pharma, GSK, Haleon, Moderna, Pfizer, Roche and Sanofi Pasteur. SGS reports consulting for AstraZeneca, CSL Seqirus, GSK, Moderna, Novavax, Pfizer, and Sanofi. The authors report no other potential conflicts of interest.
Figures
Similar articles
-
COVID-19 pandemic dynamics in India, the SARS-CoV-2 Delta variant, and implications for vaccination.medRxiv [Preprint]. 2021 Nov 22:2021.06.21.21259268. doi: 10.1101/2021.06.21.21259268. medRxiv. 2021. Update in: J R Soc Interface. 2022 Jun;19(191):20210900. doi: 10.1098/rsif.2021.0900. PMID: 34845460 Free PMC article. Updated. Preprint.
-
Real-world effectiveness and factors associated with effectiveness of inactivated SARS-CoV-2 vaccines: a systematic review and meta-regression analysis.BMC Med. 2023 Apr 27;21(1):160. doi: 10.1186/s12916-023-02861-3. BMC Med. 2023. PMID: 37106390 Free PMC article.
-
Facing the Omicron variant-how well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review.Front Immunol. 2022 Aug 24;13:940562. doi: 10.3389/fimmu.2022.940562. eCollection 2022. Front Immunol. 2022. PMID: 36091023 Free PMC article.
-
Protection of the third-dose and fourth-dose mRNA vaccines against SARS-CoV-2 Omicron subvariant: a systematic review and meta-analysis.BMJ Open. 2023 Dec 20;13(12):e076892. doi: 10.1136/bmjopen-2023-076892. BMJ Open. 2023. PMID: 38128943 Free PMC article.
-
Effectiveness of inactivated COVID-19 vaccines against SARS-CoV-2 infections among healthcare personnel in Pakistan: a test-negative case-control study.BMJ Open. 2023 Jun 27;13(6):e071789. doi: 10.1136/bmjopen-2023-071789. BMJ Open. 2023. PMID: 37369396 Free PMC article.
References
-
- Belongia EA, Simpson MD, King JP, Sundaram ME, Kelley NS, Osterholm MT, et al. Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. Lancet Infect Dis. 2016;16(8):942–51. - PubMed
-
- Chemaitelly2 H, Yassine HM, Benslimane FM, Al Khatib HA, Tang P, Hasan MR, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021;27(9):1614–21. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
