Cost-effectiveness analyses of amivantamab plus lazertinib and lazertinib versus osimertinib in non-small cell lung cancer with EGFR mutations
- PMID: 40385479
- PMCID: PMC12081244
- DOI: 10.3389/fphar.2025.1527614
Cost-effectiveness analyses of amivantamab plus lazertinib and lazertinib versus osimertinib in non-small cell lung cancer with EGFR mutations
Abstract
Background: The combination of amivantamab and lazertinib has demonstrated clinically significant and sustained antitumor effects in both treatment-naïve and osimertinib-pretreated advanced non-small cell lung cancer (NSCLC) patients harboring previously untreated epidermal growth factor receptor (EGFR) mutations.
Objectives: A cost-effectiveness analysis was conducted to compare three therapeutic strategies, namely, amivantamab with lazertinib combination therapy, lazertinib monotherapy, and osimertinib monotherapy, for advanced NSCLC patients with EGFR mutations; the patients included both treatment-naïve individuals and those previously treated with osimertinib.
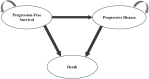
Methods: Based on a previous multicenter randomized double-blind phase III trial (NCT04487080) for evaluating amivantamab-lazertinib versus osimertinib in EGFR-mutated advanced NSCLC patients (both treatment-naïve and osimertinib-pretreated), we constructed a Markov model for 3-week cycles over a 5-year horizon. The primary outcomes of the model included total costs, quality-adjusted life years (QALYs), and incremental cost-effectiveness ratio (ICER), where all economic parameters were discounted at 3.0% annually. The cost-utility analyses employed China's per capita gross domestic product for 2023 (ranging from $12,295.7 to $36,887.0) as the willingness-to-pay (WTP) threshold supplemented by comprehensive sensitivity and scenario analyses to verify the model robustness.
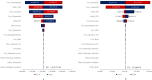
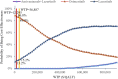
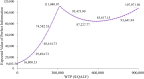
Results: The economic evaluations demonstrated that compared to osimertinib monotherapy, the amivantamab-lazertinib combination yielded an additional 1.11 QALYs at an incremental cost of $1,342,374, producing an ICER of $1,211,236/QALY that substantially exceeds the $36,887 WTP threshold. Similarly, lazertinib monotherapy showed a QALY gain of 0.71 with $224,248 of additional costs (ICER = $315,640/QALY), also surpassing the lower threshold of $12,296. The sensitivity analysis showed that the predominant model driver was drug acquisition costs.
Conclusion: The economic analyses indicate that neither amivantamab-lazertinib combination therapy nor lazertinib monotherapy represents a cost-effective first-line option for EGFR exon 20 insertion-positive NSCLC compared to osimertinib monotherapy. The substantial drug acquisition costs are the primary contributors to the unfavorable economic profiles of these treatments. Hence, future clinical implementations should carefully weigh the considerable therapeutic benefits against the significant financial burdens to achieve an optimal risk-benefit equilibrium.
Keywords: amivantamab; cost-effectiveness analysis; lazertinib; non-small cell lung cancer; osimertinib.
Copyright © 2025 Zhang, Li, Liu, Li and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A cost-effectiveness analysis of amivantamab plus lazertinib versus osimertinib in the treatment of US and Chinese patients with EGFR-mutated advanced non-small cell lung cancer.Lung Cancer. 2025 May;203:108533. doi: 10.1016/j.lungcan.2025.108533. Epub 2025 Apr 7. Lung Cancer. 2025. PMID: 40220717
-
MARIPOSA: phase 3 study of first-line amivantamab + lazertinib versus osimertinib in EGFR-mutant non-small-cell lung cancer.Future Oncol. 2022 Feb;18(6):639-647. doi: 10.2217/fon-2021-0923. Epub 2021 Dec 16. Future Oncol. 2022. PMID: 34911336
-
Plain language summary of first-line amivantamab-lazertinib in previously untreated high-risk EGFR-altered non-small-cell lung cancer in MARIPOSA.Future Oncol. 2025 May;21(12):1429-1444. doi: 10.1080/14796694.2025.2487407. Epub 2025 Apr 24. Future Oncol. 2025. PMID: 40270251 Free PMC article.
-
Comparing the Effectiveness and Safety of First-line Interventions in Patients With Advanced Epidermal Growth Factor Receptor-mutant Non-small Cell Lung Cancer, With Particular Focus on Brain Metastatic Status: A Systematic Review and Network Meta-analysis.Clin Oncol (R Coll Radiol). 2025 Apr;40:103776. doi: 10.1016/j.clon.2025.103776. Epub 2025 Jan 30. Clin Oncol (R Coll Radiol). 2025. PMID: 39951884
-
Amivantamab in the Treatment of Metastatic NSCLC: Patient Selection and Special Considerations.Onco Targets Ther. 2022 Oct 12;15:1197-1210. doi: 10.2147/OTT.S329095. eCollection 2022. Onco Targets Ther. 2022. PMID: 36246734 Free PMC article. Review.
References
-
- Bray F., Laversanne M., Sung H., Ferlay J., Siegel R. L., Soerjomataram I., et al. (2024a). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74 (3), 229–263. Epub 2024 Apr 4. PMID: 38572751. 10.3322/caac.21834 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

