Randomized, double-blind, placebo-controlled pilot study of metformin as an adjunctive therapy in Parkinson's disease
- PMID: 40385486
- PMCID: PMC12081955
- DOI: 10.3389/fphar.2025.1497261
Randomized, double-blind, placebo-controlled pilot study of metformin as an adjunctive therapy in Parkinson's disease
Abstract
Background: Parkinson's disease (PD) is caused by the progressive loss of dopaminergic neurons in the substantia nigra. Neuroinflammation is considered a key factor contributing to the pathophysiology of PD. Current gold-standard therapies for PD provide only symptomatic relief without slowing disease progression, highlighting the need to develop new disease-modifying treatments. Metformin has been demonstrated to exert a neuroprotective role in several neurodegenerative disorders including PD.
Aim: This study aimed to clarify the role of metformin as adjuvant therapy in patients with PD.
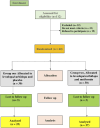
Methods: Sixty patients with PD were divided into 2 groups (n = 30). Patients in group 1 received levodopa/carbidopa (250/25 mg) three times daily for 3 months plus placebo (Control group), while those in group 2 received levodopa/carbidopa (250/25 mg) three times daily and 500 mg metformin two times daily (Metformin group). Patients were assessed via Unified Parkinson's Disease Rating Scale (UPDRS). The serum concentrations of toll like receptor 4 (TLR-4), α-synuclein, brain derived neurotropic factor (BDNF), and high mobility group box 1 (HMGB-1) were measured before and after treatment.
Primary outcome: The improvement in UPDRS from baseline to 3 months.
Secondary outcome: Change in the level of biological markers.
Results: The control group did not show significant difference in UPDRS when compared to their baseline value by Wilcoxon test (P > 0.05), meanwhile the metformin group showed significant difference when compared to before treatment by Wilcoxon test (P < 0.05). There were no significant differences between the two groups in UPDRS after treatment (P > 0.05) by Man Whitney test. However, the metformin group showed a significant decrease in TLR-4, HMGB-1, and α-synuclein along with a statistically significant increase in BDNF (P < 0.05) when compared to its baseline and control group. The control group did not show any significant changes in all markers when compared to their baseline.
Conclusion: While no significant differences in UPDRS scores were observed between the metformin and control groups, trends in biomarker changes suggest a potential impact of adjunctive metformin use on the underlying pathophysiology of PD. Further studies are needed to assess its effects on motor symptoms over a longer duration.
Clinical trial registration: identifier NCT05781711.
Keywords: Parkinson disease; TLR-4; metformin; neuro-inflammation; α-synuclein.
Copyright © 2025 AlRasheed, Bahaa, Elmasry, Elberri, Kotkata, El Sabaa, Elmorsi, Kamel, Negm, Hamouda, Aldossary, Salahuddin, Yasser, Eldesouqui, Hamouda, Eltantawy, Elawady and Abdallah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abdelgaied M. Y., Rashad M. H., El-Tayebi H. M., Solayman M. H. (2024). The impact of metformin use on the outcomes of relapse-remitting multiple sclerosis patients receiving interferon beta 1a: an exploratory prospective phase II open-label randomized controlled trial. J. Neurology 271, 1124–1132. 10.1007/s00415-023-12113-2 - DOI - PubMed
-
- Alarfaj S., Bahaa M., Yassin H., El-Khateeb E., Kotkata F., El-Gammal M., et al. (2023). A randomized placebo-controlled, double-blind study to investigate the effect of a high oral loading dose of cholecalciferol in non-alcoholic fatty liver disease patients, new insights on serum STAT-3 and hepassocin. Eur. Rev. Med. & Pharmacol. Sci. 27, 7607–7619. 10.26355/eurrev_202308_33413 - DOI - PubMed
-
- Alarfaj S. J., Bahaa M. M., Elmasry T. A., Elberri E. I., El-Khateeb E., Hamouda A. O., et al. (2024). Fenofibrate as an adjunct therapy for ulcerative colitis: targeting inflammation via SIRT1, NLRP3, and AMPK pathways: a randomized controlled pilot study. Drug Des. Dev. Ther. 18, 5239–5253. 10.2147/DDDT.S490772 - DOI - PMC - PubMed
-
- Aldossary K. M., Ali L. S., Abdallah M. S., Bahaa M. M., Elmasry T. A., Elberri E. I., et al. (2024). Effect of a high dose atorvastatin as added-on therapy on symptoms and serum AMPK/NLRP3 inflammasome and IL-6/STAT3 axes in patients with major depressive disorder: randomized controlled clinical study. Front. Pharmacol. 15, 1381523. 10.3389/fphar.2024.1381523 - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical