Synthesis, target analysis, and cerebroprotective effects of novel imide antioxidants via the Nrf2/HO-1 pathway in cerebral ischemia-reperfusion injury
- PMID: 40385488
- PMCID: PMC12081345
- DOI: 10.3389/fphar.2025.1552717
Synthesis, target analysis, and cerebroprotective effects of novel imide antioxidants via the Nrf2/HO-1 pathway in cerebral ischemia-reperfusion injury
Abstract
Background: Cerebral ischemia-reperfusion injury (CIRI) is a secondary brain injury that occurs after thrombolysis and is a primary cause of death in ischemic stroke patients. Antioxidants that effectively reduce oxidative stress are an efficient treatment approach for CIRI. Here, a novel diimide compound was synthesized using the chemical structure of previously designed anti-inflammatory skeletons.
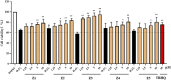
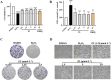
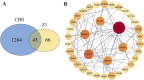
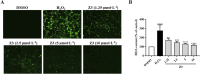
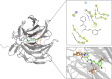
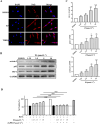
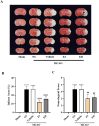
Methods and results: The antioxidant activities of five compounds (Z1-Z5) were preliminarily evaluated using the hydrogen peroxide-induced PC12 cell damage model, of which Z3 exhibited the best antioxidant effect, even exceeding that of the positive control (tert-butylhydroquinone). Enrichment analysis using network targeting and network pharmacology methods predicted seven candidate core target genes of Z3 in CIRI. Of these targets, computer molecular docking analysis predicted that Z3 has the strongest binding affinity for nuclear factor erythroid 2-related factor (Nrf2). MTT and colony formation assays, reactive oxygen species analysis, immunofluorescence, and immunoblotting experiments verified that Z3 reduced reactive oxygen species to play a protective antioxidant role via the Nrf2/hemoxygenase 1 (HO-1) pathway. The protective effect of Z3 in vivo was explored through TTC staining and neurobehavioral scoring of CIRI model mice.
Conclusion: This study provides a new drug development strategy and candidate drug for the treatment of CIRI, offering ideas for the design of new antioxidants.
Keywords: Nrf2 signaling pathway; antioxidant; cerebral ischemia-reperfusion injury; molecular docking; network pharmacology; oxidative stress.
Copyright © 2025 Huang, Chen, Zhou, Chen, Wu, Wu, Liu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
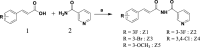

References
-
- Adamic K., Dunn M., Ingold K. U. (1969). Formation of diphenyl nitroxide in diphenylamine inhibited autoxidations. Can. J. Chem. 47 (2), 287–294. 10.1139/v69-040 - DOI
LinkOut - more resources
Full Text Sources

