Stem cell therapies in the clinic
- PMID: 40385529
- PMCID: PMC12079495
- DOI: 10.1002/btm2.70000
Stem cell therapies in the clinic
Abstract
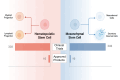
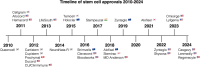
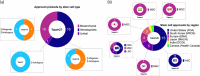
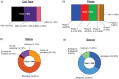
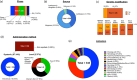
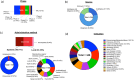
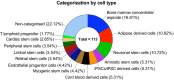
Stem cell therapies have emerged as a transformative approach in modern medicine, with the potential to address and possibly cure a broad spectrum of diseases. These therapies utilize living stem cells that can perform complex biological functions not replicable by traditional drugs. Stem cell therapies have an expanding therapeutic landscape, with several products already approved and numerous clinical trials underway. Among the various stem cell types, hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) are most widely studied. In this review, we provide a detailed analysis of the current clinical landscape of stem cell therapies. We review 27 stem cell products that have received regulatory approvals and discuss 800 ongoing clinical trials, with a focus on HSCs and MSCs. We also discuss the critical challenges to be addressed to facilitate the clinical translation of stem cell therapies.
Keywords: HSC; MSC; cell; cell therapy; clinical translation; clinical trials; iPSC; stem cell.
© 2025 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
SM and ZZ are inventors on patent applications in the field of cell therapies (owned and managed by Harvard University).
Figures

References
-
- US FDA . Yescarta—Package Insert and Medication Guide. Published online 2024. Accessed December 23, 2024. https://www.fda.gov/media/108377/download
-
- US FDA . AMTAGVI—Package Inset and Medication Guide. Published online 2024. Accessed December 23, 2024. https://www.fda.gov/media/176417/download
Publication types
LinkOut - more resources
Full Text Sources

