Milk extracellular vesicles: A burgeoning new presence in nutraceuticals and drug delivery
- PMID: 40385535
- PMCID: PMC12079498
- DOI: 10.1002/btm2.10756
Milk extracellular vesicles: A burgeoning new presence in nutraceuticals and drug delivery
Abstract
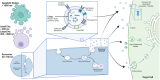
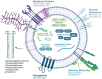
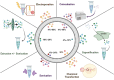
Mammalian milk, a multifaceted developmental biofluid, has attracted new attention due to its diverse constituents and their implications for health and disease. Among these constituents, extracellular vesicles (EVs) have emerged as focal points of investigation. EVs, including exosomes and small EVs, have demonstrated biological activity in preclinical studies-including reports of enhancement of cognition and neural complexity, promotion of gastrointestinal development, barrier function and microbiome richness, the bolstering of immune response, and facilitation of musculoskeletal maturation in neonates. The richness of milk as a source of EVs is noteworthy, with hundreds of milliliters (at >1012 EVs/mL) of nanovesicles extractable from a single liter of milk (>1014 EVs/starting liter of milk). Techniques such as tangential flow filtration hold promise for scalable production, potentially extending to thousands of liters. Together with the scale and increasing sophistication of the dairy industry, the abundance of EVs in milk underscores their commercial potential in various nutraceutical applications. Beyond natural bioactivity, milk EVs (mEVs) present intriguing possibilities as orally deliverable, non-immunogenic pharmaceutical carriers, with burgeoning interest in their utilization for heart disease and cancer chemotherapy and as vectors for gene-editing modules such as CrispR. This review synthesizes current knowledge on mEV biogenesis, characterization, isolation methodologies, and cargo contents. Moreover, it delves into the therapeutic potential of mEVs, both as inherently bioactive nanovesicles and as versatile platforms for drug delivery. As efforts progress toward large-scale implementation, rigorous attention to safe, industrial-scale production and robust assay development will be pivotal in harnessing the translational promise of small EVs from milk.
Keywords: drug delivery; exosome; extracellular vesicle; infant development; milk; nutraceutical; pharmaceutical.
© 2025 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
SRM and RGG are company officers at The Tiny Cargo Company, a corporation commercializing milk EV technologies.
Figures

Similar articles
-
Novel Protocols for Scalable Production of High Quality Purified Small Extracellular Vesicles from Bovine Milk.Nanotheranostics. 2021 Jul 5;5(4):488-498. doi: 10.7150/ntno.62213. eCollection 2021. Nanotheranostics. 2021. PMID: 34367882 Free PMC article.
-
Biocompatibility of highly purified bovine milk-derived extracellular vesicles.J Extracell Vesicles. 2018 Feb 21;7(1):1440132. doi: 10.1080/20013078.2018.1440132. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29511463 Free PMC article.
-
Extracellular vesicles and exosome-like nanovesicles as pioneering oral drug delivery systems.Front Bioeng Biotechnol. 2024 Jan 8;11:1307878. doi: 10.3389/fbioe.2023.1307878. eCollection 2023. Front Bioeng Biotechnol. 2024. PMID: 38260737 Free PMC article. Review.
-
Comparison of colostrum and milk extracellular vesicles small RNA cargo in water buffalo.Sci Rep. 2024 Aug 3;14(1):17991. doi: 10.1038/s41598-024-67249-6. Sci Rep. 2024. PMID: 39097641 Free PMC article.
-
Milk Extracellular Vesicles: Natural Nanoparticles for Enhancing Oral Drug Delivery against Bacterial Infections.ACS Biomater Sci Eng. 2024 Apr 8;10(4):1988-2000. doi: 10.1021/acsbiomaterials.3c01824. Epub 2024 Mar 26. ACS Biomater Sci Eng. 2024. PMID: 38529792 Review.
References
-
- Ibrahim SA, Khan YS. Histology, extracellular vesicles. StatPearls [Internet]. StatPearls Publishing; 2023. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

