Catalpol promotes the generation of cerebral organoids with oRGs through activation of STAT3 signaling
- PMID: 40385540
- PMCID: PMC12079450
- DOI: 10.1002/btm2.10746
Catalpol promotes the generation of cerebral organoids with oRGs through activation of STAT3 signaling
Abstract
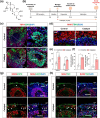
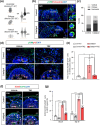
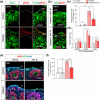
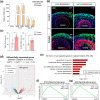
The generation of human cortical organoids containing outer radial glia (oRG) cells is crucial for modeling neocortical development. Here we show that Catalpol, an iridoid glucoside derived from Rehmannia glutinosa, significantly enhances the generation of cerebral organoids with expanded oRG populations and increased neurogenic potential. Catalpol-treated organoids exhibited thicker ventricular zone/subventricular zone (VZ/SVZ) and outer subventricular zone (oSVZ) regions, with increased numbers of SOX2 + HOPX+ and SOX2 + TNC+ oRG cells and elevated expression of oRG markers HOPX and FAM107A. We found that Catalpol promoted oRG generation through non-vertical divisions of ventricular radial glia (vRG) cells, indicating enhanced oRG generation via asymmetrical divisions. Furthermore, we demonstrated that Catalpol augmented oRG cell numbers through activation of the STAT3 signaling pathway. These findings highlight Catalpol's potential in promoting the generation of cerebral organoids with expanded oRG populations and increased neurogenic potential through STAT3 activation, offering new insights into neocortical development modeling.
Keywords: Catalpol; STAT3 signaling; cerebral organoids; outer radial glia cells.
© 2024 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Generation of human cerebral organoids with a structured outer subventricular zone.Cell Rep. 2024 Apr 23;43(4):114031. doi: 10.1016/j.celrep.2024.114031. Epub 2024 Apr 6. Cell Rep. 2024. PMID: 38583153 Free PMC article.
-
Generation of human cerebral organoids with a structured outer subventricular zone.bioRxiv [Preprint]. 2023 Feb 17:2023.02.17.528906. doi: 10.1101/2023.02.17.528906. bioRxiv. 2023. Update in: Cell Rep. 2024 Apr 23;43(4):114031. doi: 10.1016/j.celrep.2024.114031. PMID: 36824730 Free PMC article. Updated. Preprint.
-
IGF-1 signaling pathway activation promotes axonal regeneration and repair: A mechanism study on catalpol-induced functional recovery after ischemic stroke.J Ethnopharmacol. 2025 May 28;348:119808. doi: 10.1016/j.jep.2025.119808. Epub 2025 Apr 15. J Ethnopharmacol. 2025. PMID: 40245961
-
Protective effects of catalpol on cardio-cerebrovascular diseases: A comprehensive review.J Pharm Anal. 2023 Oct;13(10):1089-1101. doi: 10.1016/j.jpha.2023.06.010. Epub 2023 Jun 21. J Pharm Anal. 2023. PMID: 38024856 Free PMC article. Review.
-
Neuronal Delamination and Outer Radial Glia Generation in Neocortical Development.Front Cell Dev Biol. 2021 Feb 5;8:623573. doi: 10.3389/fcell.2020.623573. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33614631 Free PMC article. Review.
Cited by
-
Ethanol exposure impaired mitotic division in apical radial glial cells and disrupted early cortical development in human forebrain organoids: Implications for ethanol-induced microcephaly.Toxicology. 2025 Nov;517:154244. doi: 10.1016/j.tox.2025.154244. Epub 2025 Jul 28. Toxicology. 2025. PMID: 40729939
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

