Mesenchymal stem cell extracellular vesicle vascularization bioactivity and production yield are responsive to cell culture substrate stiffness
- PMID: 40385541
- PMCID: PMC12079338
- DOI: 10.1002/btm2.10743
Mesenchymal stem cell extracellular vesicle vascularization bioactivity and production yield are responsive to cell culture substrate stiffness
Abstract
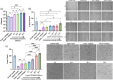
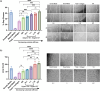
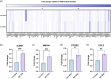
Mesenchymal stem cell-derived extracellular vesicles (MSC EVs) are an attractive therapeutic option for regenerative medicine applications due to their inherently pro-angiogenic and anti-inflammatory properties. However, reproducible and cost-effective production of highly potent therapeutic MSC EVs is challenging, limiting their translational potential. Here, we investigated whether the well-characterized responsiveness of MSCs to their mechanical environment-specifically, substrate stiffness-could be exploited to generate EVs with increased therapeutic bioactivity without the need for biochemical priming or genetic manipulation. Using polydimethylsiloxane and bone marrow-derived MSCs (BM-MSCs), we show that decreasing the stiffness of MSC substrates to as low as 3 kPa significantly improves the pro-angiogenic bioactivity of EVs as measured by tube formation and gap closure assays. We also demonstrate that lower substrate stiffness improves EV production and overall yield, important for clinical translation. Furthermore, we establish the mechanoresponsiveness of induced pluripotent stem cell-derived MSC (iMSC) EVs and their comparability to BM-MSC EVs, again using tube formation and gap closure assays. With this data, we confirm iMSCs' feasibility as an alternative, renewable cell source for EV production with reduced donor variability. Overall, these results suggest that utilizing substrate stiffness is a promising, simple, and a potentially scalable approach that does not require exogenous cargo or extraneous reagents to generate highly potent pro-angiogenic MSC EVs.
Keywords: cell‐derived therapy; exosome; mesenchymal stromal cell.
© 2024 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of The American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

