Amelioration of biased neuronal differentiation in humanized mouse model of valproic acid-induced autism by precisely targeted transcranial magnetic stimulation
- PMID: 40385547
- PMCID: PMC12079372
- DOI: 10.1002/btm2.10748
Amelioration of biased neuronal differentiation in humanized mouse model of valproic acid-induced autism by precisely targeted transcranial magnetic stimulation
Abstract
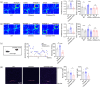
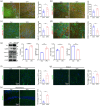
Autism spectrum disorder (ASD) is a group of developmental diseases, which still lacks effective treatments. Pregnant exposure of Valproic acid (VPA) is an important environmental risk factor for ASD, but it's long-term effects on the development of human neural cells, particularly in vivo, and the corresponding treatment have yet been fully investigated. In the present study, we first made a humanized ASD mouse model by transplanting VPA-pretreated human neural progenitor cells (hNPCs) into the cortex of immune-deficient mice. In comparison with wild type and control chimeric mice, ASD chimeric mice (VPAhNPC mice) exhibit core syndromes of ASD, namely dramatic reduction of sociability, social interaction and social communication, and remarkable increase of stereotype repetitive behaviors and anxiety-like behaviors. At cellular level, VPA-pretreatment biased the differentiation of human excitatory neurons and their axonal projections in host brain. Chemogenetic suppression of human neuronal activity restored most behavior abnormalities of VPAhNPC mice. Further, specific modulation of human neurons by a newly developed transcranial magnetic stimulation (TMS) device which could precisely target hPNCs effectively recued the core syndromes of ASD-like behaviors, restored the excitatory-inhibitory neuronal differentiation and axonal projection, and reversed the expression of over half of the VPA-affected genes. These data demonstrated that VPAhNPC mice could be used as a humanized model of ASD and that precisely targeted TMS could ameliorate the VPA-biased human neuronal differentiation in vivo.
Keywords: autism spectrum disorder; humanized mouse; precisely targeted TMS; valproic acid.
© 2025 The Author(s). Bioengineering & Translational Medicine published by Wiley Periodicals LLC on behalf of American Institute of Chemical Engineers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources

