Kidney transplant in patients with C3 glomerulopathy
- PMID: 40385590
- PMCID: PMC12082085
- DOI: 10.1093/ckj/sfaf134
Kidney transplant in patients with C3 glomerulopathy
Abstract
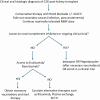
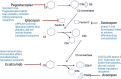
Complement protein 3 (C3) glomerulopathy (C3G) is a rare and progressive kidney disease primarily affecting young individuals and frequently advancing to end-stage kidney disease (ESKD). For ESKD, kidney transplantation remains the optimal treatment option; however, C3G has a high recurrence rate post-transplantation, affecting over two-thirds of transplanted patients. Despite advances in our understanding of C3G, significant gaps persist regarding the optimal timing for transplantation and the best strategies for peri-transplant management. Currently, no clear evidence links functional complement levels to the risk of post-transplant recurrence. Genetic counseling is also complex, due to variable gene penetrance and weak genotype-phenotype correlations, which limit predictive accuracy. Transplant-related factors are believed to significantly influence C3G recurrence, yet there are no established methods for preventing recurrence after transplantation. Eculizumab has shown inconsistent efficacy in managing recurrent C3G. However, new proximal complement inhibitors, such as factor B and C3 inhibitors, are under investigation in clinical trials and show promise. Some of these trials include kidney transplant patients with C3G, and their outcomes could potentially shape future treatment protocols.
Keywords: C3 glomerulopathy; alternative complement pathway; eculizumab; kidney transplant; recurrent disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
K.D.J. reports consultancy agreements with Novartis, Otsuka, Calliditas, Appelis, George Clinicals, Vera therapeutics, Citrus Oncology, Decipher, GSK, PMV pharmaceutics and Travere; reports honoraria from the American Society of Nephrology and the International Society of Nephrology; is a paid contributor to UpToDate.com and is section editor for Onconephrology for Nephrology Dialysis Transplantation; serves on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Frontiers in Nephrology, Journal of Onco-Nephrology and Kidney International; serves as the Editor-in-Chief of ASN Kidney News; and serves as Co-President of the American Society of Onco-Nephrology. P.S. reports consulting agreements with Appelis, Travere, Otsuka and Vera therapeutics. G.A. is the Galdi fellow in Glomerular Diseases and Onconephrology at Northwell with grant support from Greg and Linda Galdi. The remaining authors have no relevant disclosures.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

