Musculoskeletal adverse events reported post-hepatitis B vaccination in the vaccine adverse event reporting system
- PMID: 40385631
- PMCID: PMC12081253
- DOI: 10.3389/fpubh.2025.1560973
Musculoskeletal adverse events reported post-hepatitis B vaccination in the vaccine adverse event reporting system
Abstract
Introduction: Hepatitis B virus (HBV) is a major cause of chronic liver disease. While the hepatitis B vaccine has been proven effective in preventing HBV infection, concerns regarding Events Supposedly Attributable to Vaccination or Immunization (ESAVI) persist. This study aims to utilize the Vaccine Adverse Event Reporting System (VAERS) database to explore potential associations between the hepatitis B vaccine and musculoskeletal system AEs, providing a scientific basis for vaccine safety evaluations.
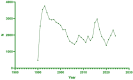
Methods: This study analyzed VAERS data from 1990 to 2024, focusing on 76,887 reports associated with hepatitis B vaccines. Disproportionality analysis methods, such as the Proportional Reporting Ratio (PRR) and Reporting Odds Ratio (ROR), were applied to identify the distribution and signal strength of musculoskeletal-related AEs. Furthermore, multivariable logistic regression analysis was conducted to explore association between patients with HBV vaccine and death.
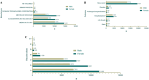
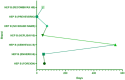
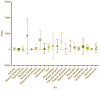
Results: Musculoskeletal system ESAVIs constituted a significant portion of all reports, including tendon fibrosis (ROR = 251.82), myofascitis (ROR = 107.51), fasciitis (ROR = 71.52), and osteoarthritis (ROR = 7.56). Tendon fibrosis demonstrated the strongest association, potentially linked to chronic inflammatory responses and abnormal tissue repair induced by aluminum adjuvants. Most AEs occurred within 30 days post-vaccination, though some, such as myofascitis, had a longer mean onset time (1,671 days), reflecting the slow-release properties of aluminum adjuvants. In multivariable logistic regression analysis, we concluded that male and combination vaccine treatment were risk factors while age from18-64 years was a protective factors of death.
Conclusion: This study identifies potential associations between hepatitis B vaccination and musculoskeletal system AEs, emphasizing the need for thorough pre-vaccination assessments and post-vaccination monitoring for high-risk individuals.
Keywords: HBV; VAERS; hepatitis B; musculoskeletal; vaccine.
Copyright © 2025 Sun, Zhu, Li, Luo and Bian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Assessing the safety of hepatitis B vaccination during pregnancy in the Vaccine Adverse Event Reporting System (VAERS), 1990-2016.Vaccine. 2018 Jan 2;36(1):50-54. doi: 10.1016/j.vaccine.2017.11.039. Epub 2017 Nov 27. Vaccine. 2018. PMID: 29174107 Free PMC article.
-
Safety of currently licensed hepatitis B surface antigen vaccines in the United States, Vaccine Adverse Event Reporting System (VAERS), 2005-2015.Vaccine. 2018 Jan 25;36(4):559-564. doi: 10.1016/j.vaccine.2017.11.079. Epub 2017 Dec 11. Vaccine. 2018. PMID: 29241647
-
A real-world pharmacovigilance analysis of hepatitis B vaccine using the U.S. Vaccine Adverse Event Reporting System (VAERS) database.Sci Rep. 2025 Feb 19;15(1):6022. doi: 10.1038/s41598-025-90135-8. Sci Rep. 2025. PMID: 39972053 Free PMC article.
-
A case-series of adverse events, positive re-challenge of symptoms, and events in identical twins following hepatitis B vaccination: analysis of the Vaccine Adverse Event Reporting System (VAERS) database and literature review.Clin Exp Rheumatol. 2004 Nov-Dec;22(6):749-55. Clin Exp Rheumatol. 2004. PMID: 15638050 Review.
-
A review of hepatitis B vaccination.Expert Opin Drug Saf. 2003 Mar;2(2):113-22. doi: 10.1517/14740338.2.2.113. Expert Opin Drug Saf. 2003. PMID: 12904111 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

