Beyond the Cure: Navigating Hepatocellular Risk and Surveillance after Hepatitis C Eradication in the Direct-acting Antiviral Era
- PMID: 40385945
- PMCID: PMC12078169
- DOI: 10.14218/JCTH.2024.00499
Beyond the Cure: Navigating Hepatocellular Risk and Surveillance after Hepatitis C Eradication in the Direct-acting Antiviral Era
Abstract
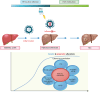
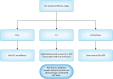
Direct-acting antivirals (DAAs) have dramatically changed the landscape of chronic hepatitis C virus (HCV) treatment and significantly reduced the risk of HCV-related hepatocellular carcinoma (HCC) after achieving sustained virologic response. However, the risk of HCC persists, particularly in patients with pre-treatment cirrhosis or fibrosis stage 3 (F3), even after DAA-induced viral eradication. While professional guidelines agree on the need for surveillance in cirrhotic patients, there is no consensus regarding surveillance for the pre-treatment F3 population following HCV eradication. The risk of HCC in the F3 population falls below the threshold for cost-effective surveillance. However, co-existing risk factors-such as diabetes, hepatic steatosis, alcohol use, advanced age, and elevated alpha-fetoprotein levels-may warrant reconsideration of HCC surveillance in this group. This underscores the need for an individualized, risk-based approach to HCC surveillance. This review provided a simplified algorithm to assist clinicians in managing patients with HCV after DAA-induced sustained virologic response.
Keywords: Cirrhosis; Direct-acting antivirals; Hepatic fibrosis; Hepatitis C virus; Hepatocellular carcinoma; Sustained virologic response.
© 2025 Authors.
Conflict of interest statement
AKS has been an Associate Editor of Journal of Clinical and Translational Hepatology since 2016. The other author has no conflict of interests related to this publication.
Figures
Similar articles
-
AGA Clinical Practice Update on Interaction Between Oral Direct-Acting Antivirals for Chronic Hepatitis C Infection and Hepatocellular Carcinoma: Expert Review.Gastroenterology. 2019 Jun;156(8):2149-2157. doi: 10.1053/j.gastro.2019.02.046. Epub 2019 Mar 13. Gastroenterology. 2019. PMID: 30878469 Free PMC article. Review.
-
Dynamics of cytokines predicts risk of hepatocellular carcinoma among chronic hepatitis C patients after viral eradication.World J Gastroenterol. 2022 Jan 7;28(1):140-153. doi: 10.3748/wjg.v28.i1.140. World J Gastroenterol. 2022. PMID: 35125824 Free PMC article.
-
Direct-acting antivirals after successful treatment of early hepatocellular carcinoma improve survival in HCV-cirrhotic patients.J Hepatol. 2019 Aug;71(2):265-273. doi: 10.1016/j.jhep.2019.03.027. Epub 2019 Apr 6. J Hepatol. 2019. PMID: 30959157
-
Risk of hepatocellular carcinoma and fibrosis evolution in hepatitis C patients with severe fibrosis or cirrhosis treated with direct acting antiviral agents.Acta Gastroenterol Belg. 2021 Jan-Mar;84(1):25-32. doi: 10.51821/84.1.420. Acta Gastroenterol Belg. 2021. PMID: 33639690
-
New perspectives in hepatocellular carcinoma surveillance after hepatitis C virus eradication.Gastroenterol Rep (Oxf). 2024 Sep 23;12:goae085. doi: 10.1093/gastro/goae085. eCollection 2024. Gastroenterol Rep (Oxf). 2024. PMID: 39319076 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
