A refined set of RxNorm drug names for enhancing unstructured data analysis in drug safety surveillance
- PMID: 40386037
- PMCID: PMC12083459
- DOI: 10.3389/ebm.2025.10374
A refined set of RxNorm drug names for enhancing unstructured data analysis in drug safety surveillance
Abstract
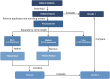
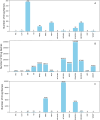
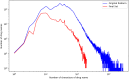
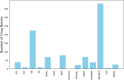
Adverse drug events are harms associated with drug use, whether the drug is used correctly or incorrectly. Identifying adverse drug events is vital in pharmacovigilance to safeguard public health. Drug safety surveillance can be performed using unstructured data. A comprehensive and accurate list of drug names is essential for effective identification of adverse drug events. While there are numerous sources for drug names, RxNorm is widely recognized as a leading resource. However, its effectiveness for unstructured data analysis in drug safety surveillance has not been thoroughly assessed. To address this, we evaluated the drug names in RxNorm for their suitability in unstructured data analysis and developed a refined set of drug names. Initially, we removed duplicates, the names exceeding 199 characters, and those that only describe administrative details. Drug names with four or fewer characters were analyzed using 18,000 drug-related PubMed abstracts to remove names which rarely appear in unstructured data. The remaining names, which ranged from five to 199 characters, were further refined to exclude those that could lead to inaccurate drug counts in unstructured data analysis. We compared the efficiency and accuracy of the refined set with the original RxNorm set by testing both on the 18,000 drug-related PubMed abstracts. The results showed a decrease in both computational cost and the number of false drug names identified. Further analysis of the removed names revealed that most originated from only one of the 14 sources. Our findings suggest that the refined set can enhance drug identification in unstructured data analysis, thereby improving pharmacovigilance.
Keywords: DrugBank; adverse drug events; database; natural language processing; pharmacovigilance.
Copyright © 2025 Guo, Dong, Liu, Aslam, Patterson and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- U.S. Food and Drug Administration. Questions and answers on FDA’s adverse event reporting system (FAERS). Available online at: https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-advers... (Accessed January 8, 2024).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

