Endothelial TIE2 Mutation Induced Contraction Deficiency of Vascular Smooth Muscle Cells via Phenotypic Transition Regulation in Venous Malformations
- PMID: 40386049
- PMCID: PMC12080580
- DOI: 10.7150/ijms.102700
Endothelial TIE2 Mutation Induced Contraction Deficiency of Vascular Smooth Muscle Cells via Phenotypic Transition Regulation in Venous Malformations
Abstract
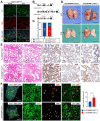
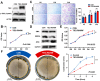
Introduction: Venous malformations (VMs) are congenital vascular malformations characterized by venous cavity enlargement and malformation. Although TIE2 mutation is a recognized genetic landscape in VMs, the regulatory role of TIE2 in vascular smooth muscle cell (VSMC) contraction remains unclear. Materials and Methods: We generated Tie2-R848Wfl/fl;Tie2Cre+ and Tie2-R848Wfl/fl;AplnER+ mice through specific expression of Tie2-R848W, a typical mutation in inherited VM, in endothelial cells (ECs). Histological and transcriptome sequencing analyses were performed on vascular abnormalities in the mutant mouse model. Postnatal vascular development in vivo was studied through morphometric analysis of the retinal vasculature. Under in vitro coculture conditions, the functional abnormality of VSMCs was studied using transwell analysis, proliferation analysis, a cell contraction assay and transcriptome sequencing analysis. Markers related to the VSMC phenotypic transition were analyzed via western blotting and quantitative RT‑PCR. Results: Tie2-R848Wfl/fl;Tie2Cre+ mice developed spontaneous pulmonary vascular malformations displaying internal hemorrhage and increased vasculature with α-SMA+ enveloped VSMCs. In Tie2-R848Wfl/fl;AplnER+ mice, Tie2-R848W mutation also induced postnatal retinal vascular malformations (higher vascular density and coverage of α-SMA+ VSMCs). According to phenotypes and molecular markers (Acta2, Cnn1, Sm22a and Opn), dysregulated phenotypic transition of VSMCs might be the pathogenic basis. Under in vitro coculture condition, the decreased contractile ability of synthetic VSMCs was significant in the mutant group, while downregulated ion transmembrane transport and TNFSF10 may play substantial roles in initiating this process. Conclusion: Endothelial TIE2 mutation might induce an abnormal EC-VSMC regulatory relationship strongly associated with phenotypic transition of VSMCs. Weakened contractility and abnormal proliferation induce chronic cavity expansion and thickening of the muscle layer, which may be potential mechanism basis of VMs.
Keywords: Endothelial cell; Mouse model; Mutation; Phenotypic transition; TIE2; Vascular smooth muscle cell.; Venous malformations.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
AKT/FOXO1 axis links cross-talking of endothelial cell and pericyte in TIE2-mutated venous malformations.Cell Commun Signal. 2020 Aug 31;18(1):139. doi: 10.1186/s12964-020-00606-w. Cell Commun Signal. 2020. PMID: 32867785 Free PMC article.
-
Angiopoietin-TIE2 feedforward circuit promotes PIK3CA-driven venous malformations.Nat Cardiovasc Res. 2025 Jul;4(7):801-820. doi: 10.1038/s44161-025-00655-9. Epub 2025 May 23. Nat Cardiovasc Res. 2025. PMID: 40410415 Free PMC article.
-
STAT1 activation by venous malformations mutant Tie2-R849W antagonizes VEGF-A-mediated angiogenic response partly via reduced bFGF production.Angiogenesis. 2013 Jan;16(1):207-22. doi: 10.1007/s10456-012-9313-x. Epub 2012 Oct 21. Angiogenesis. 2013. PMID: 23086340
-
Review of the endothelial pathogenic mechanism of TIE2-related venous malformation.J Vasc Surg Venous Lymphat Disord. 2017 Sep;5(5):740-748. doi: 10.1016/j.jvsv.2017.05.001. Epub 2017 May 16. J Vasc Surg Venous Lymphat Disord. 2017. PMID: 28818232 Review.
-
Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity.Cell Signal. 2018 Dec;52:48-64. doi: 10.1016/j.cellsig.2018.08.019. Epub 2018 Aug 30. Cell Signal. 2018. PMID: 30172025 Review.
References
-
- Du Z, Liu JL, You YH, Wang LZ, He J, Zheng JW. et al. Genetic landscape of common venous malformations in the head and neck. Journal of vascular surgery Venous and lymphatic disorders. 2021;9:1007–16. e7. - PubMed
-
- Du Z, Zheng J, Zhang Z, Wang Y. Review of the endothelial pathogenic mechanism of TIE2-related venous malformation. Journal of vascular surgery Venous and lymphatic disorders. 2017;5:740–8. - PubMed
-
- Shu W, Lin Y, Hua R, Luo Y, He N, Fang L. et al. Cutaneomucosal venous malformations are linked to the TIE2 mutation in a large Chinese family. Experimental dermatology. 2012;21:456–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

