Exploring Renin-angiotensin System Genes as Novel Prognostic Biomarkers for Oral Squamous Cell Carcinoma
- PMID: 40386053
- PMCID: PMC12080578
- DOI: 10.7150/ijms.112735
Exploring Renin-angiotensin System Genes as Novel Prognostic Biomarkers for Oral Squamous Cell Carcinoma
Abstract
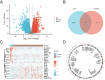
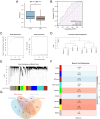
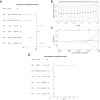
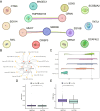
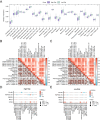
Purpose: Recent evidence suggests that the renin-angiotensin system (RAS) is involved in OSCC development. This study aimed to identify RAS-related gene (RASRG) biomarkers associated with OSCC prognosis through integrated bioinformatics analysis. Methods: First, we identified module genes by intersecting differentially expressed genes (DEGs) from the TCGA-OSCC dataset with RASRGs using weighted gene co-expression network analysis (WGCNA). Next, Cox and least absolute shrinkage and selection operator (LASSO) regression analyses were utilized to construct an OSCC risk model. We also created a nomogram incorporating risk scores and relevant clinical variables. Subsequently, receiver operating characteristic (ROC) analysis, Kaplan-Meier (KM) curve analysis, Cox regression analysis, and in vitro experiments were performed to assess the accuracy of the prognostic risk model and nomogram. Furthermore, protein-protein interaction (PPI) network, immune infiltration analysis and functional enrichment analyses were employed to reveal OSCC-related pathogenic genes and underlying mechanisms. Results: A novel OSCC risk model was established consisting of six key genes: CMA1, CTSG, OLR1, SPP1, AQP1, and PTX3. This six-gene signature effectively predicted the prognosis of patients with OSCC and served as a reliable independent prognostic parameter. Protein-protein interaction network analysis identified 5 hub genes and 13 miRNAs. Immune infiltration analysis indicated a possible association of the prognostic features of RASRGs with immunomodulation. Conclusion: In this study, we successfully constructed a risk model based on the six identified RAS-related DEGs as potential predictive biomarkers for OSCC.
Keywords: biomarkers; oral squamous cell carcinoma; prognosis; renin-angiotensin system-related genes; risk score.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. - PubMed
-
- Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

