Carboxyamidotriazole Regulates the Function of Salivary Gland Epithelial Cells and B Cells to Alleviate Experimental Sjögren's Disease in Mice
- PMID: 40386058
- PMCID: PMC12080577
- DOI: 10.7150/ijms.111897
Carboxyamidotriazole Regulates the Function of Salivary Gland Epithelial Cells and B Cells to Alleviate Experimental Sjögren's Disease in Mice
Abstract
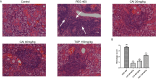
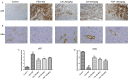
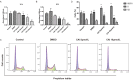
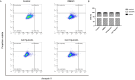
Sjögren's disease (SjD), a systemic autoimmune disease, suffers from restricted treatment choices. The activation of salivary gland epithelial cells and abnormal auto-reactive B cells, triggering cytokine and autoantibody generation, is key to its immunopathogenesis. Carboxyamidotriazole (CAI) was reported to have anti-inflammatory properties by reducing cytokines, yet its role in SjD was unknown. In this research, we targeted to probe CAI's potential treatment effect on SjD-like NOD/Ltj mice and its mechanism. Utilizing the salivary glands of these mice, we employed HE staining, ELISA, immunohistochemistry and flow cytometry. Findings revealed that CAI augmented salivary secretion, decreased water intake and serum autoantibody levels, suppressed histological alterations and lymphocyte foci, and diminished inflammatory factors such as IL-1β and IL-6. It also blocked IκBα degradation and p65 nuclear translocation. In vitro, CAI restrained IL-6 secretion from stimulated SGECs and halted Raji B cells' proliferation at G0/G1 stage. Overall, CAI shows an anti-SjD effect in NOD/Ltj mice, probably by regulating relevant cells and deactivating the NF-κB pathway.
Keywords: B cells; NOD/Ltj mice; Sjögren′s disease; carboxyamidotriazole; cytokines; salivary gland epithelial cells.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
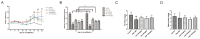

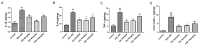
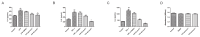
Similar articles
-
Artesunate inhibits Sjögren's syndrome-like autoimmune responses and BAFF-induced B cell hyperactivation via TRAF6-mediated NF-κB signaling.Phytomedicine. 2021 Jan;80:153381. doi: 10.1016/j.phymed.2020.153381. Epub 2020 Oct 13. Phytomedicine. 2021. PMID: 33086170
-
B7-H4 Inhibits the Development of Primary Sjögren's Syndrome by Regulating Treg Differentiation in NOD/Ltj Mice.J Immunol Res. 2020 Sep 27;2020:4896727. doi: 10.1155/2020/4896727. eCollection 2020. J Immunol Res. 2020. PMID: 33062721 Free PMC article.
-
SHP2 Allosteric Inhibitor SHP099 Alleviates Inflammation and Restores Salivary Gland Function in Sjögren's Disease-like Animals via Regulation of the IL-17RA Signaling Pathway.Int Immunopharmacol. 2025 Aug 28;161:114986. doi: 10.1016/j.intimp.2025.114986. Epub 2025 Jun 3. Int Immunopharmacol. 2025. PMID: 40466613
-
New concepts for the development of autoimmune exocrinopathy derived from studies with the NOD mouse model.Arch Oral Biol. 1999 May;44 Suppl 1:S21-5. doi: 10.1016/s0003-9969(99)00045-x. Arch Oral Biol. 1999. PMID: 10414851 Review.
-
Significance of Interleukin-6/STAT Pathway for the Gene Expression of REG Iα, a New Autoantigen in Sjögren's Syndrome Patients, in Salivary Duct Epithelial Cells.Clin Rev Allergy Immunol. 2017 Jun;52(3):351-363. doi: 10.1007/s12016-016-8570-7. Clin Rev Allergy Immunol. 2017. PMID: 27339601 Review.
References
-
- Fox RI. Sjögren's syndrome. Lancet. 2005. Jul 23-29;366(9482):321-31. doi: 10.1016/S0140-6736(05)66990-5.
-
- Balint G, Watson Buchanan W, Kean CA. et al. Sjögren's syndrome. Inflammopharmacology. 2024;32(1):37–43. doi: 10.1007/s10787-023-01222-z. - PubMed
-
- Beydon M, McCoy S, Nguyen Y. et al. Epidemiology of Sjögren syndrome. Nat Rev Rheumatol. 2024;20(3):158–169. doi: 10.1038/s41584-023-01057-6. - PubMed
-
- Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol. 2013;9(9):544–56. doi: 10.1038/nrrheum.2013.110. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

