Notch signaling pathway regulates the progression of fetal growth restriction through mediating immune dysfunction
- PMID: 40386306
- PMCID: PMC12082063
- DOI: 10.3892/br.2025.1989
Notch signaling pathway regulates the progression of fetal growth restriction through mediating immune dysfunction
Abstract
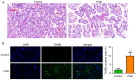
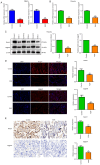
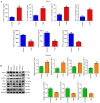
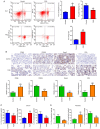
Fetal growth restriction (FGR) is associated with an increased risk of neonatal morbidity and mortality, as well as the development of metabolic syndrome in adulthood. The present study investigated the regulatory mechanisms of Notch signaling in FGR progression. The expression levels of Notch1 and Jagged1 were determined using reverse transcription-quantitative PCR, western blotting, immunofluorescence staining and immunohistochemistry (IHC). ELISA was used to measure the concentrations of IL-10, IL-17 and IL-35 in serum and placental samples. ELISA and western blotting determined the inflammation- and angiogenesis-related cytokine levels. Th17, Treg and macrophage levels were determined using IHC and flow cytometry. Additionally, hematoxylin & eosin staining and TUNEL assay assessed placenta histology and trophoblast cell apoptosis. Significant trophoblast apoptosis was observed in the placenta of FGR pregnancies. The expression of Notch1 and Jagged1 in peripheral blood mononuclear cells and placental tissues of FGR pregnancies was significantly lower than in the control group. The FGR group exhibited a remarkable inflammation, anti-angiogenesis and immune dysfunction. In conclusion, the Notch signaling pathway mediates immune balance to regulate the development of FGR. These findings offer the potential for advancing innovative predictive, diagnostic and therapeutic approaches for FGR.
Keywords: Jagged1; Notch1; fetal growth restriction; immune balance; inflammation.
Copyright: © 2025 Ye et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Formyl peptide receptor-2 is decreased in foetal growth restriction and contributes to placental dysfunction.Mol Hum Reprod. 2018 Feb 1;24(2):94-109. doi: 10.1093/molehr/gax067. Mol Hum Reprod. 2018. PMID: 29272530
-
PLAC1 Regulates the Occurrence of Fetal Growth Restriction by Inhibiting the Apoptosis of Trophoblast Cells.Ann Clin Lab Sci. 2021 Mar;51(2):182-189. Ann Clin Lab Sci. 2021. PMID: 33941557
-
Down-regulation of placental neuropilin-1 in fetal growth restriction.Am J Obstet Gynecol. 2016 Feb;214(2):279.e1-279.e9. doi: 10.1016/j.ajog.2015.09.068. Epub 2015 Sep 26. Am J Obstet Gynecol. 2016. PMID: 26409917
-
The placental blood perfusion and LINC00473-mediated promotion of trophoblast apoptosis in fetal growth restriction.Gene. 2024 Nov 15;927:148727. doi: 10.1016/j.gene.2024.148727. Epub 2024 Jun 26. Gene. 2024. PMID: 38942180
-
Mechanistic insights into the development of severe fetal growth restriction.Clin Sci (Lond). 2023 Apr 26;137(8):679-695. doi: 10.1042/CS20220284. Clin Sci (Lond). 2023. PMID: 37186255 Free PMC article. Review.
References
-
- Gupta N, Khajuria A. Histomorphological features of placenta in pregnancy complicated with intrauterine growth retardation. JK Sci. 2016;18:21–25.
-
- Turner S, Posthumus AG, Steegers EAP, AlMakoshi A, Sallout B, Rifas-Shiman SL, Oken E, Kumwenda B, Alostad F, Wright-Corker C, et al. Household income, fetal size and birth weight: An analysis of eight populations. J Epidemiol Community Health. 2022;76:629–636. doi: 10.1136/jech-2021-218112. - DOI - PubMed
-
- González-Fernández D, Muralidharan O, Neves PA, Bhutta ZA. Associations of maternal nutritional status and supplementation with fetal, newborn, and infant outcomes in low-income and middle-income settings: An overview of reviews. Nutrients. 2024;16(3725) doi: 10.3390/nu16213725. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
