This is a preprint.
HDAC11 promotes renal fibrosis by induing partial epithelial-mesenchymal transition and G2/M phase arrest in renal epithelial cells
- PMID: 40386380
- PMCID: PMC12083684
- DOI: 10.21203/rs.3.rs-6523050/v1
HDAC11 promotes renal fibrosis by induing partial epithelial-mesenchymal transition and G2/M phase arrest in renal epithelial cells
Abstract
Background: Histone deacetylase 11 (HDAC11) is the sole member of class IV HDACs, implicated in tumor growth, immune regulation, and oxidative stress injury. Its specific role in renal fibrosis and underlying mechanisms remains unclear.
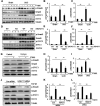
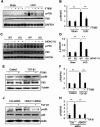
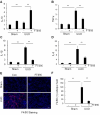
Methods: The global knockout of HDAC11 mice and FT895, a selective inhibitor of HDAC11, were utilized to assess the role of HDAC11 in renal fibrosis following unilateral ureteral obstruction (UUO) injury in mice. Immunostaining was employed to analyze renal expression of HDAC11 and infiltration of macrophages. Immunoblot analysis was used to analyze the expression and/or phosphorylation of proteins associated with partial epithelial-mesenchymal transition (pEMT) in the kidney and cultured renal proximal tubular cells (RTPCs). RT-PCR was used to analyze the expression of various proinflammatory cytokines.
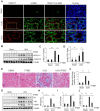
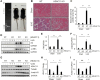
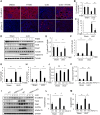
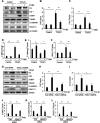
Results: HDAC11 was predominantly expressed in renal epithelial cells, with its expression increasing in the kidney following UUO. This upregulation correlated with excessive collagen deposition and was associated with increased levels of fibronectin, collagen I, and α-smooth muscle actin, alongside reduced E-cadherin expression. Both global deletion of HDAC11 and treatment with the selective inhibitor FT895 significantly reduced collagen accumulation and the expression of fibronectin and collagen I, while preserving E-cadherin levels. HDAC11 inhibition also led to a decrease in histone H3 phosphorylation at serine 10, a marker of G2/M cell cycle arrest, and reduced the expression of Snail and Twist-key transcription factors involved in pEMT. Similar effects were observed in TGFb1-stimulated renal proximal tubular cells in vitro treated with FT895 or subjected to HDAC11 silencing via siRNA. Additionally, FT895 treatment attenuated the expression of multiple pro-inflammatory cytokines and reduced macrophage infiltration in obstructed kidneys. Both pharmacological inhibition and genetic ablation of HDAC11 suppressed activation of profibrotic signaling pathways, including Smad3, STAT3, and NF-κB, in both in vitro and in vivo models.
Conclusions: These findings indicate that HDAC11 is crucial for renal fibrosis development by promoting pEMT and G2/M phase cell cycle arrest in renal epithelial cells through multiple profibrotic signaling pathways. Therefore, targeting HDAC11 may be a promising therapeutic strategy to alleviate renal fibrosis.
Keywords: FT895; Histone deacetylase 11; Smad3; kidney fibrosis; partial epithelial-mesenchymal transition; signal transducer and activator of transcription 3 Nuclear factor kappa B; unilateral ureteral obstruction.
Conflict of interest statement
Competing interests The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Identification of histone deacetylase 8 as a novel therapeutic target for renal fibrosis.FASEB J. 2020 Jun;34(6):7295-7310. doi: 10.1096/fj.201903254R. Epub 2020 Apr 12. FASEB J. 2020. PMID: 32281211 Free PMC article.
-
Blocking the histone lysine 79 methyltransferase DOT1L alleviates renal fibrosis through inhibition of renal fibroblast activation and epithelial-mesenchymal transition.FASEB J. 2019 Nov;33(11):11941-11958. doi: 10.1096/fj.201801861R. Epub 2019 Aug 2. FASEB J. 2019. PMID: 31373855 Free PMC article.
-
Blocking the class I histone deacetylase ameliorates renal fibrosis and inhibits renal fibroblast activation via modulating TGF-beta and EGFR signaling.PLoS One. 2013;8(1):e54001. doi: 10.1371/journal.pone.0054001. Epub 2013 Jan 16. PLoS One. 2013. PMID: 23342059 Free PMC article.
-
Histone Deacetylase 11 Contributes to Renal Fibrosis by Repressing KLF15 Transcription.Front Cell Dev Biol. 2020 Apr 17;8:235. doi: 10.3389/fcell.2020.00235. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32363192 Free PMC article.
-
Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis.Autophagy. 2016 Sep;12(9):1472-86. doi: 10.1080/15548627.2016.1190071. Epub 2016 Jun 15. Autophagy. 2016. PMID: 27304991 Free PMC article.
References
-
- Arts J., King P., Marien A., Floren W., Belien A., Janssen L., Pilatte I., Roux B., Decrane L., Gilissen R., et al. (2009). JNJ-26481585, a novel “second-generation” oral histone deacetylase inhibitor, shows broad-spectrum preclinical antitumoral activity. Clin Cancer Res 15, 6841–6851. - PubMed
-
- Bello A.K., Okpechi I.G., Levin A., Ye F., Damster S., Arruebo S., Donner J.A., Caskey F.J., Cho Y., Davids M.R., et al. (2024). An update on the global disparities in kidney disease burden and care across world countries and regions. Lancet Glob Health 12, e382–e395. - PubMed
-
- Castillo R.F. (2023). Pathophysiologic Implications and Therapeutic Approach of Klotho in Chronic Kidney Disease: A Systematic Review. Lab Invest 103, 100178. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

