This is a preprint.
Association of Placental Mitochondrial DNA Mutations on Infant Negative Affectivity: Modifying Effects of Maternal Lifetime Stress and Infant Sex
- PMID: 40386437
- PMCID: PMC12083669
- DOI: 10.21203/rs.3.rs-5806105/v1
Association of Placental Mitochondrial DNA Mutations on Infant Negative Affectivity: Modifying Effects of Maternal Lifetime Stress and Infant Sex
Update in
-
Association of placental mitochondrial DNA mutations on infant negative affectivity: modifying effects of maternal lifetime stress and infant sex.Biol Sex Differ. 2025 Jun 10;16(1):40. doi: 10.1186/s13293-025-00717-4. Biol Sex Differ. 2025. PMID: 40495250 Free PMC article.
Abstract
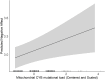
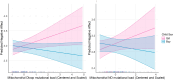
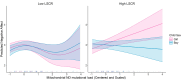
Neuropsychiatric and behavioral disorders impact over 15% of U.S. children, with sex differences in manifestation. Prenatal exposure to psychosocial stress predicts adverse neurodevelopmental outcomes, particularly during gestation. Mechanisms remain poorly understood. Research links prenatal stress exposures with placental mitochondrial DNA (mtDNA) mutational load, suggesting that disrupted mitochondrial placental function may play a role. We conceptualize that placental mitochondrial biomarkers reflect environmentally-induced oxidation that may contribute to mechanisms influencing neurodevelopment. Furthermore, as maternal stress can impact female and male children differently, this may in part explain sex differences in early childhood neurobehavioral outcomes. This study explores the association between placental mtDNA mutational load and negative affectivity in infants, and whether these associations are modified by maternal lifetime stress and fetal sex. Placenta samples (N = 394) were collected at delivery and whole mtDNA sequencing was performed to identify gene-specific mutational loads. Mothers completed the Infant Behavior Questionnaire-Revised (IBQ-R) when children were 6.69±:1.61 months of age and the Negative Affectivity factor was derived. Multivariable regression analyses were performed to model Negative Affectivity in relation to placental mtDNA mutational load, first adjusting for child sex and maternal age, self-reported race, and education. Lastly, we examined effect modification by maternal stress and fetal sex using cross-product terms and contrast statements. Results showed that higher mutational load in the MT_CYB region was positively associated with increased negative affectivity. Notably, interactions between mtDNA regions (MT_DLOOP and MT_ND), child sex, and maternal stress revealed that girls with higher mutational loads in these regions were at greater risk for increased negative affectivity, particularly under high maternal stress. These findings suggest that placental mtDNA mutational load could serve as a biomarker for neurodevelopmental risk, with sex-specific vulnerabilities influenced by maternal stress. This study underscores the importance of considering both environmental factors and sex differences in understanding early neurodevelopmental trajectories, and the potential of the placenta as a tool for early detection and intervention. Further research is needed to validate these findings and explore their implications for long-term child development.
Keywords: Placenta; maternal stress; mitochondria; negative affectivity; neurodevelopment.
Conflict of interest statement
Competing interests The authors report no biomedical financial interests or potential conflicts of interest.
Figures
Similar articles
-
Association of placental mitochondrial DNA mutations on infant negative affectivity: modifying effects of maternal lifetime stress and infant sex.Biol Sex Differ. 2025 Jun 10;16(1):40. doi: 10.1186/s13293-025-00717-4. Biol Sex Differ. 2025. PMID: 40495250 Free PMC article.
-
Associations Between Maternal Lifetime Stress and Placental Mitochondrial DNA Mutations in an Urban Multiethnic Cohort.Biol Psychiatry. 2021 Mar 15;89(6):570-578. doi: 10.1016/j.biopsych.2020.09.013. Epub 2020 Sep 18. Biol Psychiatry. 2021. PMID: 33229036 Free PMC article.
-
Prenatal PM2.5 exposure and infant temperament at age 6 months: Sensitive windows and sex-specific associations.Environ Res. 2022 Apr 15;206:112583. doi: 10.1016/j.envres.2021.112583. Epub 2021 Dec 17. Environ Res. 2022. PMID: 34922978 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm.Pediatr Res. 2021 Jan;89(2):326-335. doi: 10.1038/s41390-020-01236-1. Epub 2020 Nov 12. Pediatr Res. 2021. PMID: 33184498 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

