Multicenter, randomized controlled trial of EUS-guided fine-needle biopsy using a fork-tip needle with macroscopic or rapid on-site evaluation for pancreatic lesions (H2O trial)
- PMID: 40386452
- PMCID: PMC12080678
- DOI: 10.1097/eus.0000000000000087
Multicenter, randomized controlled trial of EUS-guided fine-needle biopsy using a fork-tip needle with macroscopic or rapid on-site evaluation for pancreatic lesions (H2O trial)
Abstract
Background and objectives: According to previous reports, EUS-fine-needle biopsy (FNB) with or without rapid on-site evaluation (ROSE) showed the nonsuperiority of EUS-FNB + ROSE over EUS-FNB. However, previous studies included various kinds of FNB needle. This might be a critical limitation due to heterogeneity. Therefore, the aim of the present multicenter, randomized controlled trial was to compare the diagnostic accuracy of the fork-tip needle with or without ROSE for pancreatic lesions.
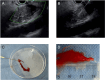
Methods: In the ROSE group, if an adequate sample was obtained to diagnose by on-site evaluation, EUS-FNB was stopped. If cytological results were insufficient or indeterminate, EUS-FNB was repeated. In the macroscopic on-site evaluation (MOSE) group, if a 4-mm length of visible core tissue was obtained, EUS-FNB was finished. If visible core tissue was not obtained or was less than 4 mm in length, a second puncture was attempted.
Results: One hundred seventy-one patients were randomized, 85 to the ROSE group and 86 to the MOSE group. In the MOSE group, diagnostic sensitivity and accuracy were 94.4% and 91.8%, respectively, for visible core tissue and 80.6% and 70.0%, respectively, for red tissue. Finally, overall diagnostic sensitivity and accuracy were 97.1% and 95.3%, respectively, in the ROSE group and 95.8% and 95.3%, respectively, in the MOSE group. Although there were no significant differences regarding adverse events between groups, the mean number of punctures was significantly lower in the MOSE group than in the ROSE group (1.47 vs. 1.20, P = 0.0171).
Conclusions: EUS-FNB using a fork-tip needle for pancreatic lesions has high diagnostic yield even without ROSE.
Keywords: EUS; EUS-FNA; EUS-guided fine-needle biopsy; Macroscopic on-site evaluation; Rapid on-site evaluation.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc on behalf of Scholar Media Publishing.
Conflict of interest statement
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Figures
References
-
- Erickson RA. EUS-guided FNA. Gastrointest Endosc 2004;60:267–279. - PubMed
-
- Kulesza P, Eltoum IA. Endoscopic ultrasound–guided fine-needle aspiration: sampling, pitfalls, and quality management. Clin Gastroenterol Hepatol 2007;5:1248–1254. - PubMed
-
- Haba S Yamao K Bhatia V, et al. . Diagnostic ability and factors affecting accuracy of endoscopic ultrasound–guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol 2013;48:973–981. - PubMed
-
- Matynia AP Schmidt RL Barraza G, et al. . Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: a systematic review and meta-analysis. J Gastroenterol Hepatol 2014;29:697–705. - PubMed
LinkOut - more resources
Full Text Sources

