Downregulation of CPT2 promotes proliferation and migration through the TNFα/NF-κB pathway in cholangiocarcinoma
- PMID: 40386589
- PMCID: PMC12078819
- DOI: 10.21037/jgo-24-685
Downregulation of CPT2 promotes proliferation and migration through the TNFα/NF-κB pathway in cholangiocarcinoma
Abstract
Background: Carnitine palmitoyltransferase II (CPT2) is an important regulatory enzyme involved in fatty acid oxidation; it is associated with the prognosis and progression of colorectal and ovarian cancers, but its expression and role in cholangiocarcinoma (CCA) have been less explored. This study aims to explore the role and molecular mechanism of CPT2 in CCA and to determine the potential relationship between CPT2 expression and the prognosis of CCA patients.
Methods: Bioinformatics analyses were used to assess CPT2 expression in CCA and other cancers. Independent prognostic factors of CCA were identified for univariate and multivariate Cox regression analyses. Nomograms were employed to predict CCA 1-, 3-, and 5-year survival. Kaplan-Meier curves explored the correlation between CPT2 expression and CCA survival. We used time-dependent receiver operating characteristics (ROCs) to evaluate the predictive efficiency of CPT2. Furthermore, potential mechanisms of CPT2 were analyzed by Gene Set Enrichment Analysis (GSEA) in CCA. CPT2 expression in peripheral blood, tissues, and cell lines of CCA was verified by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting. The effect of CPT2 on CCA cells was gauged using Cell Counting Kit-8 (CCK-8), cell cycle, apoptosis, and transwell assays. Finally, the regulation of the TNFα/NF-κB pathway by CPT2 was verified by Western blotting.
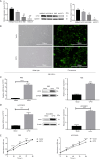
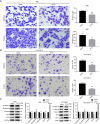
Results: CPT2 expression was down-regulated in many cancers, including CCA. COX regression analyses showed that CPT2 expression and the clinical stage could be independent prognostic factors in CCA. Nomograms indicated that the lower probability of CCA survival was associated with the lower expression of CPT2 and the higher clinical stage. The Kaplan-Meier curve showed that the low expression of CPT2 was related to a poor prognosis in CCA. The time-dependent ROC curve demonstrated the predictive ability of CPT2 [1-, 3-, 5-year are under the curve (AUC) =0.933, 0.61, 0.612]. Functionally, CPT2 overexpression inhibited CCA cell proliferation, down-regulated CDK4/6 expression to arrest CCA cells at G1, induced apoptosis by up-regulating BAX expression, cleaved-caspase-3 expression, and down-regulating Bcl2 expression, and reduced migration and invasion via suppression of epithelial-mesenchymal transition (EMT). Knocking down CPT2 showed the opposite results. Mechanistically, overexpression of CPT2 could decrease TNFα and phosphorylated p65 (p-p65; Ser536) expression and inhibit NF-κB pathway activation. CPT2 knockdown yielded opposite results.
Conclusions: CPT2 is a potential prognostic marker of CCA, a tumor suppressor gene to inhibit the malignant progression of CCA, and therefore a potential therapeutic target.
Keywords: Cholangiocarcinoma (CCA); NF-κB; bioinformatics; carnitine palmitoyltransferase II (CPT2).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-24-685/coif). The authors have no conflicts of interest to declare.
Figures






Similar articles
-
Downregulation of CPT2 promotes proliferation and inhibits apoptosis through p53 pathway in colorectal cancer.Cell Signal. 2022 Apr;92:110267. doi: 10.1016/j.cellsig.2022.110267. Epub 2022 Jan 30. Cell Signal. 2022. PMID: 35108639
-
Up-regulated LINC00261 predicts a poor prognosis and promotes a metastasis by EMT process in cholangiocarcinoma.Pathol Res Pract. 2020 Jan;216(1):152733. doi: 10.1016/j.prp.2019.152733. Epub 2019 Nov 11. Pathol Res Pract. 2020. PMID: 31812439
-
MCM2 promotes the proliferation, migration and invasion of cholangiocarcinoma cells by reducing the p53 signaling pathway.Yi Chuan. 2022 Mar 20;44(3):230-244. doi: 10.16288/j.yczz.21-426. Yi Chuan. 2022. PMID: 35307646
-
miR-378 serves as a prognostic biomarker in cholangiocarcinoma and promotes tumor proliferation, migration, and invasion.Cancer Biomark. 2019;24(2):173-181. doi: 10.3233/CBM-181980. Cancer Biomark. 2019. PMID: 30594918
-
HSDL2 knockdown promotes the progression of cholangiocarcinoma by inhibiting ferroptosis through the P53/SLC7A11 axis.World J Surg Oncol. 2023 Sep 18;21(1):293. doi: 10.1186/s12957-023-03176-6. World J Surg Oncol. 2023. PMID: 37718459 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
