Efficacy and safety of dose adjustment for fruquintinib in the third-line treatment of metastatic colorectal cancer: a retrospective study with real-world settings
- PMID: 40386609
- PMCID: PMC12078818
- DOI: 10.21037/jgo-2024-881
Efficacy and safety of dose adjustment for fruquintinib in the third-line treatment of metastatic colorectal cancer: a retrospective study with real-world settings
Abstract
Background: Fruquintinib, a standard third-line treatment option for metastatic colorectal cancer (mCRC), has been shown to significantly prolong both overall survival (OS) and progression-free survival (PFS) in patients. However, we have observed that the standard dosing of fruquintinib is frequently associated with a higher incidence of adverse effects within the Chinese population, leading some patients-particularly elderly individuals-to be unable to tolerate it. This study presents a retrospective analysis to evaluate the therapeutic efficacy and safety of adjusting the administration frequency of fruquintinib in patients with mCRC.
Methods: We conducted a retrospective analysis of the follow-up data and clinicopathological characteristics of 99 patients with mCRC who were treated with an adjusted frequency of furquinitinib administration at our center. We conduct regular imaging follow-ups and tumor marker evaluations to assess the therapeutic efficacy in patients. PFS data are collected through these assessments, while OS and adverse effects information is obtained via structured telephone follow-ups.
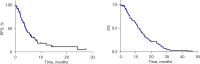
Results: There were 99 patients with mCRC treated with fruquintinib monotherapy at an adjusted dosing frequency. The median progression-free survival (mPFS) for the 99 patients on fruquintinib monotherapy was 4.1 months and the median overall survival (mOS) was 11.4 months following the adjustment of dosing frequency, the overall response rate (ORR) was 2.0%, and the disease control rate (DCR) was recorded at 40.4% within the fruquintinib monotherapy. Overall, after receiving oral administration of fruquintinib at the modified frequency, no grade 3 or higher adverse reactions occurred in all patients.
Conclusions: Our results showed that the administration of fruquintinib at an adjusted dosing frequency has not significantly impacted the efficacy while demonstrating a favorable safety profile. However, this conclusion necessitates further validation through prospective clinical trials with a larger sample size.
Keywords: Fruquintinib; dose adjustment; efficacy; metastatic colorectal cancer (mCRC); safety.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-2024-881/coif). The authors have no conflicts of interest to declare.
Figures
Comment in
-
New dosing schedules in pre-treated patients with metastatic colorectal cancer.J Gastrointest Oncol. 2025 Aug 30;16(4):1773-1778. doi: 10.21037/jgo-2025-492. Epub 2025 Aug 26. J Gastrointest Oncol. 2025. PMID: 40950335 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
