Effect of Ring Composition on the Statics and Dynamics of Block Copolyelectrolyte Catenanes
- PMID: 40386687
- PMCID: PMC12080470
- DOI: 10.1021/acs.macromol.5c00099
Effect of Ring Composition on the Statics and Dynamics of Block Copolyelectrolyte Catenanes
Abstract
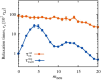
We use Langevin simulations to study the effect of ring composition on the structure and dynamics of model polycatenanes with copolyelectrolyte rings, each made of one charged and one neutral block. Key observables have a nonmonotonic dependence on ring composition, including the radius of gyration, mechanical bond length, orientational correlations, and rotational relaxation times. Microscopic analysis shows that these nonmonotonicities arise from the competition between electrostatic repulsion, pulling rings apart, and topological constraints, enforcing the proximity of neighboring rings. By locking charged-neutral interfaces at the mechanically bonded regions, this interplay can induce a strong chemical orientational order along the catenane while also hindering the local relaxation dynamics. Chemical orientation defects, manifesting as neutral-neutral interfaces, can emerge too and migrate along the catenane via coupled reorientations of neighboring rings. Our results clarify how ring composition and mechanical bonds can define the properties of topological materials across different scales.
© 2025 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


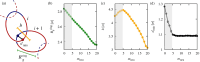
 ; the average mechanical bond length, b (defined as the distance between the centers of mass of
concatenated rings); and the average distance of minimum approach
between monomers of two concatenated rings, dmin, see sketch in panel (a). The shaded band marks the interval
0 ≤ mneu ≤ 5 where the b and dmin curves exhibit qualitatively
distinct behavior compared to longer neutral blocks.
; the average mechanical bond length, b (defined as the distance between the centers of mass of
concatenated rings); and the average distance of minimum approach
between monomers of two concatenated rings, dmin, see sketch in panel (a). The shaded band marks the interval
0 ≤ mneu ≤ 5 where the b and dmin curves exhibit qualitatively
distinct behavior compared to longer neutral blocks.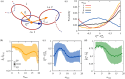


 , is the characteristic decay time of the
orientational correlation function of the catenane end-to-end vector.
The latter,
, is the characteristic decay time of the
orientational correlation function of the catenane end-to-end vector.
The latter,  is the characteristic decay time of the
orientational correlation function of the ring’s diameter vectors,
averaged over all diameters.
is the characteristic decay time of the
orientational correlation function of the ring’s diameter vectors,
averaged over all diameters.
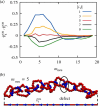
 and one of the two corresponding mechanical
bonds b̂i. The
data are for a stretch of a few consecutive rings at mneu = 5. The configurations corresponding to the two selected
times (dashed lines) are shown in panel (b) and illustrate the hopping
of the defect across neighboring rings.
and one of the two corresponding mechanical
bonds b̂i. The
data are for a stretch of a few consecutive rings at mneu = 5. The configurations corresponding to the two selected
times (dashed lines) are shown in panel (b) and illustrate the hopping
of the defect across neighboring rings.Similar articles
-
Mechanical Pulling of Linked Ring Polymers: Elastic Response and Link Localisation.Polymers (Basel). 2017 Aug 1;9(8):327. doi: 10.3390/polym9080327. Polymers (Basel). 2017. PMID: 30971003 Free PMC article.
-
Tunable Knot Segregation in Copolyelectrolyte Rings Carrying a Neutral Segment.ACS Macro Lett. 2021 Nov 16;10(11):1365-1370. doi: 10.1021/acsmacrolett.1c00453. Epub 2021 Oct 19. ACS Macro Lett. 2021. PMID: 35549022
-
Topological Effects in Isolated Poly[n]catenanes: Molecular Dynamics Simulations and Rouse Mode Analysis.ACS Macro Lett. 2018 Aug 21;7(8):938-943. doi: 10.1021/acsmacrolett.8b00393. Epub 2018 Jul 18. ACS Macro Lett. 2018. PMID: 35650969
-
Polycatenanes: synthesis, characterization, and physical understanding.Chem Soc Rev. 2022 Jun 20;51(12):4928-4948. doi: 10.1039/d2cs00256f. Chem Soc Rev. 2022. PMID: 35611843 Review.
-
Distinctive features and challenges in catenane chemistry.Chem Sci. 2022 Feb 7;13(12):3315-3334. doi: 10.1039/d1sc05391d. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432874 Free PMC article. Review.
References
-
- Hart L. F.; Hertzog J. E.; Rauscher P. M.; Rawe B. W.; Tranquilli M. M.; Rowan S. J. Material properties and applications of mechanically interlocked polymers. Nat. Rev. Mater. 2021, 6, 508–530. 10.1038/s41578-021-00278-z. - DOI
-
- Frisch H. L.; Wasserman E. Chemical Topology. J. Am. Chem. Soc. 1961, 83, 3789–3795. 10.1021/ja01479a015. - DOI
LinkOut - more resources
Full Text Sources
