Efficacy and safety of anlotinib plus EGFR tyrosine kinase inhibitors in slow- or locally progressing non-small cell lung cancer after adjuvant therapy
- PMID: 40386710
- PMCID: PMC12082220
- DOI: 10.21037/tlcr-2025-177
Efficacy and safety of anlotinib plus EGFR tyrosine kinase inhibitors in slow- or locally progressing non-small cell lung cancer after adjuvant therapy
Abstract
Background: Anlotinib, a small-molecule tyrosine kinase inhibitor (TKI), suppresses angiogenesis and tumor progression. As the mechanisms underlying the resistance to epidermal growth factor receptor (EGFR)-TKIs are complex and diverse, further exploration of new treatment strategies is necessary. Combination therapy with EGFR-TKIs and anlotinib targets multiple signaling pathways, enhancing efficacy in patients with EGFR-positive non-small cell lung cancer (NSCLC). This study evaluated the efficacy and safety of anlotinib with EGFR-TKIs in patients with NSCLC who developed resistance to postoperative adjuvant therapy.
Methods: From January 2020 to December 2023, 48 patients at the Department of Thoracic Surgery, the First Affiliated Hospital of Zhejiang University, who developed resistance to adjuvant therapy were included in this retrospective study. All patients received anlotinib (10-12 mg, po, d1-14, q3w) alongside their original EGFR-TKI regimen. The primary endpoint was progression-free survival (PFS), while secondary endpoints included 6- and 12-month PFS rates, overall survival (OS), and safety. PFS was defined as the time from the initiation of anlotinib plus EGFR-TKI to disease progression or death, and OS was defined as the time from the start of anlotinib plus EGFR-TKI to death from any cause.
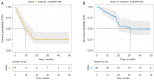
Results: Among the 48 patients, 23 previously received first- or second-generation EGFR-TKIs, and 25 received third-generation EGFR-TKIs. As of March 25, 2024, the median follow-up duration was 33.3 months [95% confidence interval (CI): 23.2-43.3]. The median PFS was 9.5 months (95% CI: 4.8-14.3), and 6- and 12-month PFS rates were 70.8% and 47.9%, respectively. For patients previously treated with first-/second- and third-generation EGFR-TKIs, the median PFS was 10.3 months (95% CI: 6.1-14.4) and 7.7 months (95% CI: 4.8-10.6), with a 6-month PFS rate of 69.6% and 72.0%, respectively, and a 12-month PFS rate of 47.8% and 48.0%, respectively. The median OS was 31.0 months [95% CI: not reached (NR)-NR], with 6-month and 12-month rates of 91.7% and 85.4%, respectively. For patients previously treated with first-/second- and third-generation EGFR-TKIs, the median OS was NR and 20.3 months (95% CI: 10.7-30.0), respectively; meanwhile, the OS rates were 95.7% and 88.0% at 6 months, and 91.3% and 80.0% at 12 months, respectively. The incidence rates of any grade and grade ≥3 treatment-related adverse events (TRAEs) were 75.0% (36/48) and 10.4% (5/48), respectively. The most common TRAEs included hypertension (17/48, 35.4%), proteinuria (15/48, 31.3%), rash (11/48, 22.9%), fatigue (5/48, 10.4%), and diarrhea (4/48, 8.3%), and no new safety events were observed. Dose reduction and discontinuation of anlotinib were reported in four (8.3%) and five (10.4%) patients previously treated with first-/second- and third-generation EGFR-TKIs, respectively.
Conclusions: Patients with NSCLC who developed resistance to postoperative EGFR-TKIs demonstrated promising efficacy and manageable safety, extending the treatment window and survival opportunities.
Keywords: Anlotinib; non-small cell lung cancer (NSCLC); overall survival (OS); progression-free survival (PFS); treatment-related adverse events (TRAEs).
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-177/coif). The authors have no conflicts of interest to declare.
Figures



Similar articles
-
A pilot study of anlotinib with third-generation epidermal growth factor receptor tyrosine kinase inhibitors in untreated EGFR-mutant patients with advanced non-small cell lung cancer.Transl Lung Cancer Res. 2023 Jun 30;12(6):1256-1263. doi: 10.21037/tlcr-23-175. Epub 2023 Jun 21. Transl Lung Cancer Res. 2023. PMID: 37425401 Free PMC article.
-
A phase II trial of anlotinib plus EGFR-TKIs in advanced non-small cell lung cancer with gradual, oligo, or potential progression after EGFR-TKIs treatment (CTONG-1803/ALTER-L001).J Hematol Oncol. 2025 Jan 5;18(1):3. doi: 10.1186/s13045-024-01656-0. J Hematol Oncol. 2025. PMID: 39757186 Free PMC article. Clinical Trial.
-
Low‑dose anlotinib combined with EGFR‑TKI can be used as an alternative for EGFR‑TKI‑resistant non‑small cell lung cancer in elderly patients.Oncol Lett. 2023 Jun 13;26(2):323. doi: 10.3892/ol.2023.13909. eCollection 2023 Aug. Oncol Lett. 2023. PMID: 37415629 Free PMC article.
-
MET tyrosine kinase inhibitors in combination with EGFR tyrosine kinase inhibitors in NSCLC patients with EGFR mutations and acquired MET alterations: a systematic review and meta-analysis.BMC Cancer. 2025 Apr 18;25(1):732. doi: 10.1186/s12885-025-14145-5. BMC Cancer. 2025. PMID: 40251527 Free PMC article.
-
Efficacy and Safety of Epidermal Growth Factor Receptor (EGFR) Inhibitors Plus Antiangiogenic Agents as First-Line Treatments for Patients With Advanced EGFR-Mutated Non-small Cell Lung Cancer: A Meta-Analysis.Front Oncol. 2020 Jun 25;10:904. doi: 10.3389/fonc.2020.00904. eCollection 2020. Front Oncol. 2020. PMID: 32714857 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
