LNCAROD was stabilized through N6-methyladenosine methylation and exerted its anticancer effects in lung squamous cell carcinoma by inhibiting SIRT1 activity via CCAR2
- PMID: 40386722
- PMCID: PMC12082203
- DOI: 10.21037/tlcr-2025-267
LNCAROD was stabilized through N6-methyladenosine methylation and exerted its anticancer effects in lung squamous cell carcinoma by inhibiting SIRT1 activity via CCAR2
Abstract
Background: Lung squamous cell carcinoma (LUSC), a deadly malignant tumor, is highly prevalent worldwide. Accumulating evidence indicates that long-chain noncoding RNAs play crucial regulatory roles in the occurrence and progression of LUSC. LNCAROD regulates the proliferation, migration, and invasion of cells by upregulating SERPINE1 expression in lung adenocarcinoma (LUAD). However, the functional mechanism of LNCAROD action in LUSC remains unclear. The aim of this study was to investigate the regulatory function and mechanism of LNCAROD action in the development of LUSC.
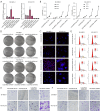
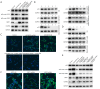
Methods: Using quantitative polymerase chain reaction (qPCR) detection, we determined the expression of LNCAROD in LUSC tissues and cell lines. Cell Counting Kit-8 (CCK-8), EdU (5-ethynyl-2'-deoxyuridine), JC-1 mitochondrial membrane potential, flow cytometry, colony formation, scratch healing, and Transwell assays were conducted, and cell proliferation, migration, and invasion, as well as physiological changes were assessed. The tumorigenicity of LUSC cells was analyzed by in vitro tumor formation in nude mice. Molecular interactions were verified via Western blotting, RNA-protein pull-down assay, RNA binding protein immunoprecipitation (RIP), N6-methyladenosine (m6A)-RIP, and coimmunoprecipitation (Co-IP) analyses.
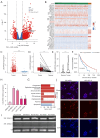
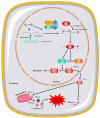
Results: LNCAROD was specifically and highly expressed in LUSC cells and tissues. LNCAROD expression was mediated by IGF2BP2 m6A methylation, which, along with CCAR2, inhibited SIRTI1's acetylation activity. This further induced p53 protein acetylation and promoted the mitochondrial apoptosis of LUSC cells, thereby inhibiting cell proliferation, migration, and invasion.
Conclusions: LNCAROD is specifically highly expressed in LUSC cells and tissues and may be a tumor-suppressor gene. The findings contribute to a deeper understanding of the function of LNCAROD in LUSC, and it may serve as a potential prognostic marker for personalized medical diagnosis in clinical practice.
Keywords: CCAR2; LNCAROD; SIRTI1; lung squamous cell carcinoma (LUSC); m6A methylation.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-267/coif). R.B. reports that his institution receives funding from Astra Zeneca, Pfizer, and Roche, and that he serves on the Advisory Board of Bayer, Boeringher Ingelheim, EISAI, Lilly, Menarini, and GSK. The other authors have no conflicts of interest to declare.
Figures




Similar articles
-
LNCAROD enhances hepatocellular carcinoma malignancy by activating glycolysis through induction of pyruvate kinase isoform PKM2.J Exp Clin Cancer Res. 2021 Sep 22;40(1):299. doi: 10.1186/s13046-021-02090-7. J Exp Clin Cancer Res. 2021. Retraction in: J Exp Clin Cancer Res. 2023 Mar 2;42(1):54. doi: 10.1186/s13046-023-02626-z. PMID: 34551796 Free PMC article. Retracted.
-
LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma.Mol Oncol. 2020 Jun;14(6):1282-1296. doi: 10.1002/1878-0261.12676. Epub 2020 Apr 13. Mol Oncol. 2020. PMID: 32216017 Free PMC article.
-
N6-methyladenosine-mediated upregulation of LNCAROD confers radioresistance in esophageal squamous cell carcinoma through stabilizing PARP1.Clin Transl Med. 2024 Oct;14(10):e70039. doi: 10.1002/ctm2.70039. Clin Transl Med. 2024. PMID: 39367700 Free PMC article.
-
IGF2BP3/CTCF Axis-Dependent NT5DC2 Promotes M2 Macrophage Polarization to Enhance the Malignant Progression of Lung Squamous Cell Carcinomas.Clin Respir J. 2024 Nov;18(11):e70031. doi: 10.1111/crj.70031. Clin Respir J. 2024. PMID: 39506204 Free PMC article.
-
RBM15 facilities lung adenocarcinoma cell progression by regulating RASSF8 stability through N6 Methyladenosine modification.Transl Oncol. 2024 Aug;46:102018. doi: 10.1016/j.tranon.2024.102018. Epub 2024 Jun 4. Transl Oncol. 2024. PMID: 38838436 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
