Comprehensive management of MET tyrosine kinase inhibitor-induced peripheral edema in patients with MET-altered non-small-cell lung cancer: a narrative review
- PMID: 40386723
- PMCID: PMC12082197
- DOI: 10.21037/tlcr-24-866
Comprehensive management of MET tyrosine kinase inhibitor-induced peripheral edema in patients with MET-altered non-small-cell lung cancer: a narrative review
Abstract
Background and objective: The mesenchymal-epithelial transition factor (MET) proto-oncogene plays an important role in the development of non-small cell lung cancer (NSCLC). MET tyrosine kinase inhibitors (TKIs) have shown promising antitumor activity in patients with NSCLC harboring MET alterations. Peripheral edema (PE), the most common adverse event of MET TKIs, has received increasing attention from clinicians. The aim of this review is to describe the incidence, potential molecular mechanisms, diagnosis, and management of MET TKI-induced PE, to increase the recognition and standardize the management of PE.
Methods: We conducted a comprehensive literature search across PubMed, Wanfang Med Online, China National Knowledge Infrastructure (CNKI), and the oncology conferences websites for related studies published between 2000 and 2023. Of the 491 titles screened, we identified 80 research articles fitting the inclusion criteria and a comprehensive literature review was conducted. The review incorporated patient conditions, comprehensive examinations, and clinical experiences to propose a standardized management framework.
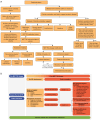
Key content and findings: The review focused on the incidence of MET TKI-induced PE, its potential molecular mechanisms, diagnostic criteria, and management strategies. The etiology of edema is complex in cancer patients; however, it may involve treatment-related increases in vascular permeability, impacts on renal function, and hypoalbuminemia. Based on the literature review, a diagnostic and comprehensive management approach for MET TKI-induced PE is proposed, which includes prevention strategies, non-pharmacological treatments, pharmacological interventions, and dosage adjustments related to MET TKIs.
Conclusions: In this review, we propose a diagnostic and comprehensive management approach for MET TKI-induced PE. By standardizing management, clinicians can enhance patient care for those treated with MET TKIs, facilitating earlier detection of PE, reducing patient suffering, and improving treatment adherence and outcomes.
Keywords: MET tyrosine kinase inhibitor (MET TKI); Non-small cell lung cancer (NSCLC); adverse event (AE); peripheral edema (PE); safety management.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-866/coif). Y.L.W. reports the grants from AstraZeneca, BMS, and Pfizer and speaker fees from Roche, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Sanofi, MSD, Hengrui, Pfizer, and BMS. The other authors have no conflicts of interest to declare.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
