Tumor microenvironment and immune-related myositis: addressing muscle wasting in cancer immunotherapy
- PMID: 40386783
- PMCID: PMC12081358
- DOI: 10.3389/fimmu.2025.1580108
Tumor microenvironment and immune-related myositis: addressing muscle wasting in cancer immunotherapy
Abstract
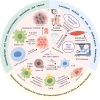
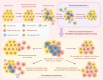
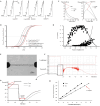
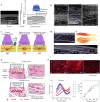
Cancer immunotherapy, which leverages the immune system to target neoplastic cells, has undergone significant transformation in recent. However, immunotherapy may have negative effects on skeletal muscle function, causing muscle wasting and functional decline in cancer patients. In this study, we review the mechanisms by which immunotherapy influences skeletal muscle, focusing on immune-related myositis, inflammation, and metabolic alterations within the tumor microenvironment (TME). The key methodologies, including biomechanical assessment techniques such as electrical impedance myography and ultrasound imaging, are discussed to provide valuable insights into process that maintain muscle integrity and function in patients receiving immunotherapy. Moreover, the dual effects of immunotherapy on tumor suppression and muscle damage are described, revealing the significance of inflammatory cytokines, immune checkpoints, and metabolic disturbances within the TME. Importantly, we propose combination therapies integrating immunotherapy and nutritional interventions or anti-inflammatory interventions as potential approaches for mitigating muscle wasting. This study highlights the need for deeper investigations to optimize immunotherapy and improve its efficacy in preserving muscle health, thereby improving patient outcomes and quality of life.
Keywords: cancer immunotherapy; inflammatory cytokines; muscle wasting; skeletal muscle; tumor microenvironment.
Copyright © 2025 Ma, Zhao, Sui, Chen, Wu, Wang, Xu, Lu, Wang, Wu, Wu, Liu and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

