Single-fluorogen imaging reveals distinct environmental and structural features of biomolecular condensates
- PMID: 40386802
- PMCID: PMC12084160
- DOI: 10.1038/s41567-025-02827-7
Single-fluorogen imaging reveals distinct environmental and structural features of biomolecular condensates
Abstract
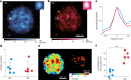
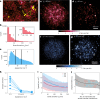
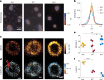
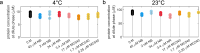
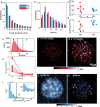
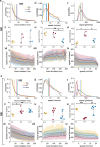
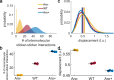
Biomolecular condensates are viscoelastic materials. Simulations predict that condensates formed by intrinsically disordered proteins are network fluids defined by spatially inhomogeneous organization of the underlying molecules. Here, we test these predictions and find that molecules within condensates are organized into slow-moving nanoscale clusters and fast-moving dispersed molecules. These results, obtained using single-fluorogen tracking and super-resolution imaging of different disordered protein-based condensates, affirm the predicted spatially inhomogeneous organization of molecules within condensates. We map the internal environments and interfaces of condensates using fluorogens that localize differently to the interiors versus interface between dilute phase and condensate. We show that nanoscale clusters within condensates are more hydrophobic than regions outside the clusters, and regions within condensates that lie outside clusters are more hydrophobic than coexisting dilute phases. Our findings provide a structural and dynamical basis for the viscoelasticity of condensates.
Keywords: Imaging and sensing; Nanoscale biophysics.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe pixOL microscope used in this work was invented by T.W. and M.D.L. and is covered by US patent 11994470 B2 (2024), which was filed by and assigned to Washington University in St. Louis. R.V.P. is a member of the scientific advisory board of and a shareholder at Dewpoint Therapeutics Inc. The work reported here was not influenced by this affiliation. The remaining authors declare no competing interests.
Figures







Update of
-
Single fluorogen imaging reveals distinct environmental and structural features of biomolecular condensates.bioRxiv [Preprint]. 2024 Oct 7:2023.01.26.525727. doi: 10.1101/2023.01.26.525727. bioRxiv. 2024. Update in: Nat Phys. 2025;21(5):778-786. doi: 10.1038/s41567-025-02827-7. PMID: 36747818 Free PMC article. Updated. Preprint.
Similar articles
-
Single fluorogen imaging reveals distinct environmental and structural features of biomolecular condensates.bioRxiv [Preprint]. 2024 Oct 7:2023.01.26.525727. doi: 10.1101/2023.01.26.525727. bioRxiv. 2024. Update in: Nat Phys. 2025;21(5):778-786. doi: 10.1038/s41567-025-02827-7. PMID: 36747818 Free PMC article. Updated. Preprint.
-
Biomolecular Condensates are Characterized by Interphase Electric Potentials.J Am Chem Soc. 2024 Oct 16;146(41):28268-28281. doi: 10.1021/jacs.4c08946. Epub 2024 Oct 2. J Am Chem Soc. 2024. PMID: 39356108
-
Biomolecular condensates are characterized by interphase electric potentials.bioRxiv [Preprint]. 2024 Jul 4:2024.07.02.601783. doi: 10.1101/2024.07.02.601783. bioRxiv. 2024. Update in: J Am Chem Soc. 2024 Oct 16;146(41):28268-28281. doi: 10.1021/jacs.4c08946. PMID: 39005320 Free PMC article. Updated. Preprint.
-
Conformational Dynamics of Intrinsically Disordered Proteins Regulate Biomolecular Condensate Chemistry.Chem Rev. 2022 Mar 23;122(6):6719-6748. doi: 10.1021/acs.chemrev.1c00774. Epub 2022 Feb 18. Chem Rev. 2022. PMID: 35179885 Free PMC article. Review.
-
Biomolecular condensates in cancer biology.Cancer Sci. 2022 Feb;113(2):382-391. doi: 10.1111/cas.15232. Epub 2021 Dec 14. Cancer Sci. 2022. PMID: 34865286 Free PMC article. Review.
Cited by
-
Condenzymes: Biomolecular condensates with inherent catalytic activities.bioRxiv [Preprint]. 2025 May 16:2024.07.06.602359. doi: 10.1101/2024.07.06.602359. bioRxiv. 2025. PMID: 39026887 Free PMC article. Preprint.
-
Quantitative spatial analysis of chromatin biomolecular condensates using cryoelectron tomography.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2426449122. doi: 10.1073/pnas.2426449122. Epub 2025 May 6. Proc Natl Acad Sci U S A. 2025. PMID: 40327693 Free PMC article.
-
Nanoscale domains govern local diffusion and aging within FUS condensates.Res Sq [Preprint]. 2025 Apr 14:rs.3.rs-6406576. doi: 10.21203/rs.3.rs-6406576/v1. Res Sq. 2025. PMID: 40321778 Free PMC article. Preprint.
-
Tunable metastability of condensates reconciles their dual roles in amyloid fibril formation.bioRxiv [Preprint]. 2025 Mar 22:2024.02.28.582569. doi: 10.1101/2024.02.28.582569. bioRxiv. 2025. Update in: Mol Cell. 2025 Jun 5;85(11):2230-2245.e7. doi: 10.1016/j.molcel.2025.05.011. PMID: 38464104 Free PMC article. Updated. Preprint.
-
Structured protein domains enter the spotlight: modulators of biomolecular condensate form and function.Trends Biochem Sci. 2025 Mar;50(3):206-223. doi: 10.1016/j.tibs.2024.12.008. Epub 2025 Jan 17. Trends Biochem Sci. 2025. PMID: 39827079 Review.
References
-
- Jawerth, L. et al. Protein condensates as aging Maxwell fluids. Science370, 1317–1323 (2020). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
