UTX Responds to Nanotopography to Suppress Macrophage Inflammatory Response by Remodeling H3K27me3 Modification
- PMID: 40386877
- PMCID: PMC12362724
- DOI: 10.1002/advs.202505723
UTX Responds to Nanotopography to Suppress Macrophage Inflammatory Response by Remodeling H3K27me3 Modification
Abstract
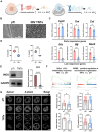
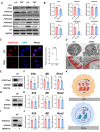
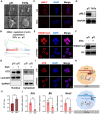
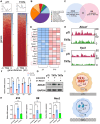
Peri-implantitis is the leading cause of implant failure, primarily due to weak defense at the implant-soft tissue interface, which disrupts the local immune microenvironment. As an integral part of this microenvironment, the implant-tissue interface plays a critical role in shaping immune cell function. Thus, engineering the surface topography of implants has emerged as a novel strategy for sustained immunomodulation following implantation. This study investigated the mechanical regulation of macrophage function by nanopatterned topographies. Titanium nanotubes (TNTs) surfaces reduce the expression of phosphorylated myosin light chain (pMLC) and promote the retention of the UTX histone methyltransferase in the nucleus. This process attenuates the enrichment of the repressive H3K27me3 histone marker at the Abca1 gene locus, increasing Abca1 expression and suppressing inflammation. This study reveals the mechanosensitivity of UTX and provides a new target for the development of therapeutic strategies that integrate mechanical signaling and immune modulation.
Keywords: histone methylation; macropahge; mechano‐immunology; nanotopography.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
H3K27me3 Demethylase UTX Restrains Plasma Cell Formation.J Immunol. 2022 Apr 15;208(8):1873-1885. doi: 10.4049/jimmunol.2100948. Epub 2022 Mar 28. J Immunol. 2022. PMID: 35346967 Free PMC article.
-
MLL1 histone methyltransferase and UTX histone demethylase functionally cooperate to regulate the expression of NRF2 in response to ROS-induced oxidative stress.Free Radic Biol Med. 2024 May 1;217:48-59. doi: 10.1016/j.freeradbiomed.2024.03.018. Epub 2024 Mar 23. Free Radic Biol Med. 2024. PMID: 38527695
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Microbial Dysbiosis, Titanium Release, and Peri-implantitis.J Dent Res. 2025 May;104(5):473-480. doi: 10.1177/00220345241307939. Epub 2025 Feb 14. J Dent Res. 2025. PMID: 39953673 Review.
-
Interventions for replacing missing teeth: different types of dental implants.Cochrane Database Syst Rev. 2014 Jul 22;(7):CD003815. doi: 10.1002/14651858.CD003815.pub4. Cochrane Database Syst Rev. 2014. PMID: 25048469
References
MeSH terms
Substances
Grants and funding
- 82371018/National Natural Science Foundation of China
- U23A20447/National Natural Science Foundation of China
- 82170936/National Natural Science Foundation of China
- KJZD-K202300402/Scientific and Technological Research Program of Chongqing Municipal Education Commission
- W0079/Program for Youth Innovation in Future Medicine, Chongqing Medical University
- cqmudstd202203/Project of Chongqing Medical University Graduate Tutor Team
- CSTB2024NSCQ-MSX0525/Natural Science Foundation of Chongqing
- 2024MD764058/China Postdoctoral Science Foundation
- KQY202302/Postgraduate Research and Innovation Project of the Affiliated Stomatological Hospital of Chongqing Medical University
- 2023CQBSHTB3129/Special Grant for Chongqing Postdoctoral Research Project
LinkOut - more resources
Full Text Sources
Research Materials
