Modulation of TTR gene expression in the eye using modified siRNAs
- PMID: 40386915
- PMCID: PMC12086539
- DOI: 10.1093/nar/gkaf409
Modulation of TTR gene expression in the eye using modified siRNAs
Abstract
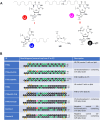
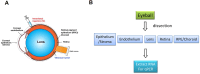
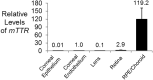
Small interfering RNAs (siRNAs) are a proven therapeutic approach for controlling gene expression in the liver. Expanding the clinical potential of RNA interference requires developing strategies to enhance delivery to extra-hepatic tissues. In this study, we examine inhibiting transthyretin (TTR) gene expression by siRNAs in the eye. Anti-TTR siRNAs have been developed as successful drugs to treat TTR amyloidosis. When administered systemically, anti-TTR siRNAs alleviate symptoms by blocking TTR expression in the liver. However, TTR amyloidosis also affects the eye, suggesting a need for reducing ocular TTR gene expression. Here, we demonstrate that pyrimidine C5- and 2'-O-linked lipid-modified siRNAs formulated in saline can inhibit TTR expression in the eye when administered locally by intravitreal injection. Modeling suggests that length and accessibility of the lipid chains contribute to in vivo silencing. GalNAc-modified siRNAs also inhibit TTR expression, albeit less potently. These data support lipid-modified siRNAs as an approach to treating the ocular consequences of TTR amyloidosis. Inhibition of TTR expression throughout the eye demonstrates that lipid-siRNA conjugates have the potential to be a versatile platform for ocular drug discovery.
© The Author(s) 2025. Published by Oxford University Press on behalf of Nucleic Acids Research.
Conflict of interest statement
M.M., J.K., and D.D. are employees of Alnylam Pharmaceuticals. V.V.M. is the Paul T. Stoffel/Centex Professor in Clinical Care. D.R.C. is the Rusty Kelley Professor of Biomedical Science. V.V.M., D.R.C., and J.H. hold a patent related to the use of dsRNAs to treat Fuch’s endothelial corneal dystrophy. D.R.C. holds the position of Executive Editor for Nucleic Acids Research and has not peer reviewed or made any editorial decisions for this paper.
Figures






Update of
-
Modulation of TTR Gene Expression in the Eye using Modified Duplex RNAs.bioRxiv [Preprint]. 2025 Mar 13:2025.03.11.642595. doi: 10.1101/2025.03.11.642595. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 May 10;53(9):gkaf409. doi: 10.1093/nar/gkaf409. PMID: 40161828 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

