Mapping of Functional Metabolic Phenotypes in Acute Myeloid Leukemia
- PMID: 40386930
- PMCID: PMC12086641
- DOI: 10.1002/cam4.70950
Mapping of Functional Metabolic Phenotypes in Acute Myeloid Leukemia
Abstract
Background: Acute myeloid leukemia (AML) is an aggressive hematologic malignancy with a poor prognosis, particularly in older patients. AML is highly heterogeneous, influenced by various chromosomal, genetic, and epigenetic alterations.
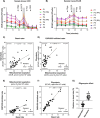
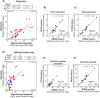
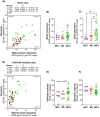
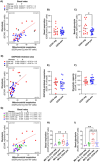
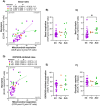
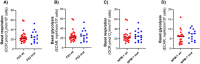
Methods: This study investigated the metabolic profiles of primary AML cells from 46 patients, focusing on mitochondrial respiration and glycolysis. We hypothesized that the metabolic profiles would reflect distinct disease characteristics. Using Seahorse technology, we measured the oxygen consumption rate (OCR) for mitochondrial respiration and the extracellular acidification rate (ECAR) for glycolysis.
Results: Our results showed significant variability in metabolic activity, with some samples relying more on glycolysis than mitochondrial respiration. Mature AML cells (FAB M4/M5, CD34 negative) exhibited increased rates of both mitochondrial respiration and glycolysis, indicating distinct metabolic adaptations. Higher glycolytic activity was observed in patients with adverse cytogenetic abnormalities. However, no clear associations were found between metabolic profiles and mutations in FLT3 or NPM1.
Conclusion: These findings highlight the role of metabolic variability in AML and suggest that targeting specific metabolic pathways could offer therapeutic opportunities, particularly for subgroups like FAB M4/M5 with unique metabolic features. Further studies are needed to refine these therapeutic strategies for clinical application.
Keywords: acute myeloid leukemias; glycolysis; metabolic phenotypes; metabolism.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
H.R. has consulted Novartis and Glaxo Smith Kline. The other authors declare no conflicts of interest.
Figures

References
-
- DiNardo C. D., Erba H. P., Freeman S. D., and Wei A. H., “Acute Myeloid Leukaemia,” Lancet 401, no. 10393 (2023): 2073–2086. - PubMed
-
- Extermann M., Artz A., Rebollo M. A., et al., “Treating Acute Myelogenous Leukemia in Patients Aged 70 and Above: Recommendations From the International Society of Geriatric Oncology (SIOG),” Journal of Geriatric Oncology 15, no. 2 (2024): 101626. - PubMed
-
- Reikvam H., “Revisiting the Prognostic Role of FLT3 Mutations in Acute Myelogenous Leukemia,” Expert Review of Hematology 16, no. 5 (2023): 317–323. - PubMed
-
- Falini B., Martelli M. P., Brunetti L., Gjertsen B. T., and Andresen V., “The NPM1 Mutant Defines AML Irrespective of Blast Count,” American Journal of Hematology 98, no. 7 (2023): E187–E189. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

