Implementing Negative Control Outcomes to Assess Comparability of Treatments for Psoriasis and Psoriatic Arthritis
- PMID: 40387023
- PMCID: PMC12087269
- DOI: 10.1002/pds.70156
Implementing Negative Control Outcomes to Assess Comparability of Treatments for Psoriasis and Psoriatic Arthritis
Abstract
Purpose: Treatment selection is typically associated with prognosis, leading to potential confounding in comparative studies. We used negative control outcomes (NCOs) to identify potential residual confounding when comparing apremilast initiators to other psoriasis (PsO) or psoriatic arthritis (PsA) treatment initiators.
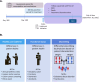
Methods: Adults with PsO/PsA who initiated treatment from September 23/March 21, 2016, respectively, with apremilast, topicals, methotrexate, interleukin (IL)-17 inhibitor (i), IL-12/23i, or tumor necrosis factor inhibitor (TNFi) were identified in the OPTUM Clinformatics DataMart database. Follow-up ended at treatment switch/discontinuation, NCO, end of enrollment, or September 30, 2022. NCOs addressed confounding for healthy users (wellness visit, herpes zoster vaccine, colon cancer screening, pelvic screening), functional status (accidents), and channeling. The 1-year relative risk (RR) for each NCO was estimated for all treatment comparisons using an inverse probability of treatment and censoring weighted estimator.
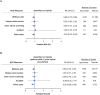
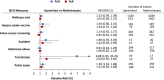
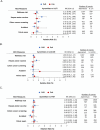
Results: In PsO, potential healthy user bias was detected in apremilast vs. IL-17i initiators, with a higher likelihood of herpes zoster vaccine and colon cancer screening (RR [95% CI]: 2.01 [1.41, 2.88] and 1.42 [1.13, 1.77], respectively). Wellness visits and pelvic exams were less likely among apremilast vs. topical initiators (0.84 [0.72, 0.98] and 0.83 [0.70, 0.98], respectively). The wellness visit RR was attenuated in individuals with ≥ 1 pre-index topical prescription (0.90 [0.78, 1.04]). In PsA, minimal residual confounding was observed between apremilast and other treatments.
Conclusions: Eligibility criteria (prior topicals) and weighting reduced residual confounding when comparing apremilast vs. other treatments for PsO and PsA. Integration of NCOs into comparative effectiveness/safety studies of PsO/PsA treatments may help identify unmeasured confounding.
Keywords: apremilast; biologics; methotrexate; negative control outcome; psoriasis; psoriatic arthritis; topicals.
© 2025 Amgen and The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
M.E.H.: Consultant for Amgen Inc. A.O.: AbbVie, Amgen Inc., BMS, Janssen, Novartis, and Pfizer—grant/research support; AbbVie, Amgen Inc., Bristol Myers Squibb, CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry, Eli Lilly, Gilead, GSK, Janssen, Novartis, Pfizer, UCB, and Takeda—consultant. K.K.O., S.T.K., K.V.T., C.D., and M.C.: Employee and stockholder of Amgen Inc. M.A.B.: American Academy of Allergy, Asthma & Immunology, Amgen, Atara Biotherapeutics, Brigham and Women's Hospital, Gilead, Intercept, National Institute of Diabetes and Digestive and Kidney Diseases, Regeneron, and Target RWE, Vertex—scientific advisory committee; Accompany Health and VitriVax—equity.
Figures
References
-
- McInnes I. B., Sawyer L. M., Markus K., LeReun C., Sabry‐Grant C., and Helliwell P. S., “Targeted Systemic Therapies for Psoriatic Arthritis: A Systematic Review and Comparative Synthesis of Short‐Term Articular, Dermatological, Enthesitis and Dactylitis Outcomes,” RMD Open 8, no. 1 (2022): e002074, 10.1136/rmdopen-2021-002074. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

