Approach to the Evaluation and Management of Interstitial Lung Abnormalities: An Official American Thoracic Society Clinical Statement
- PMID: 40387336
- PMCID: PMC12264694
- DOI: 10.1164/rccm.202505-1054ST
Approach to the Evaluation and Management of Interstitial Lung Abnormalities: An Official American Thoracic Society Clinical Statement
Erratum in
-
Erratum: Approach to the Evaluation and Management of Interstitial Lung Abnormalities: An Official American Thoracic Society Clinical Statement.Am J Respir Crit Care Med. 2025 Sep;211(9):1727. doi: 10.1164/rccm.v211erratum4. Am J Respir Crit Care Med. 2025. PMID: 40879359 Free PMC article. No abstract available.
Abstract
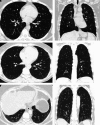
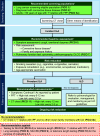
Background: There is growing interest in identifying early stages of interstitial lung disease (ILD) to improve patient outcomes. This document reviews updated evidence on interstitial lung abnormalities (ILAs); provides suggestions for screening, evaluation, and management; proposes criteria for distinguishing ILAs from ILD; and identifies research priorities. Methods: A committee of clinical and methodology experts met by video conference to define ILAs and ILD by consensus and voted on 11 prespecified questions after reviewing synthesized evidence from a systematic literature search. Agreement of ≥70% was required to approve each suggestion. Results: ILA is defined as nondependent bilateral parenchymal abnormalities on computed tomography, including ground-glass opacities or reticulations, lung distortion, traction bronchiectasis, and/or honeycombing involving ≥5% of a lung zone. The updated definition removes the prior exclusion of high-risk populations. ILD is distinguished from ILAs by symptoms (dyspnea/cough) attributable to an interstitial process, abnormal or declining lung function, fibrotic (honeycombing and/or reticulation with traction bronchiectasis involving ≥5% of total lung volume) or progressive imaging abnormalities, and/or specific fibrotic ILD patterns on imaging or pathology. Suggestions include ILA/ILD assessment on imaging acquired for lung cancer screening, screening adults with connective tissue disease and first-degree relatives of patients with familial pulmonary fibrosis, assessing baseline symptoms and pulmonary function among those with ILAs, and monitoring ILAs with chest computed tomography every 2-3 years. Conclusions: This document presents a comprehensive literature review of ILAs with updates to the Fleischner Society ILA definition, establishes a working ILD definition, and provides evidence-based suggestions for ILA evaluation and management.
Keywords: computed tomography; interstitial lung abnormalities (ILA); interstitial lung disease (ILD); pulmonary fibrosis; screening.
Figures

References
-
- Mirza RD, Bolster MB, Johnson SR, Allen A, Jr, Bernstein EJ, Chung JH. et al. Assessing patient values and preferences to inform the 2023 American College of Rheumatology/American College of Chest Physicians interstitial lung disease guidelines. Arthritis Care Res (Hoboken) . 2024;76:1083–1089. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

