Mycobacterium tuberculosis bacillus induces pyroptosis in human lung fibroblasts
- PMID: 40387341
- PMCID: PMC12188705
- DOI: 10.1128/msphere.00110-25
Mycobacterium tuberculosis bacillus induces pyroptosis in human lung fibroblasts
Abstract
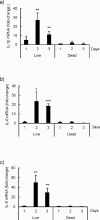
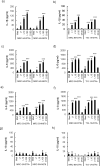
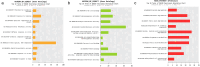
We previously reported that live, but not dead, virulent Mycobacterium tuberculosis (Mtb) H37Rv bacilli induce cell death in human lung fibroblast cell lines, MRC-5, MRC-9, and TIG-1. Here, using two distinct Mtb strains from two different lineages (HN878 lineage 2 and H37Rv lineage 4), we confirmed cell death at day 2 after infection with a device that measures cell growth/cytotoxicity in real time (Maestro-Z [AXION]). Mtb bacilli uptake by the fibroblast was confirmed with a transmission electron microscope on day 2. Expressions of inflammatory cytokines and interleukin (IL)-1β, IL-6, and IL-8 were observed when exposed to live, but not dead bacteria. The cell death of fibroblasts induced by both Mtb strains tested was prevented by caspase-1/4 and NLRP3 inflammasome inhibitors, but not by caspase-3 and caspase-9 inhibitors. Therefore, we classified the fibroblast cell death by Mtb infection as pyroptosis. To investigate the biological and pathological relevance of fibroblast cell death by Mtb infection, we performed dual RNA-Seq analysis on Mtb within fibroblasts and Mtb-infected fibroblasts at day 2. In Mtb bacilli tcrR, secE2, ahpD, and mazF8 genes were highly induced during infection. These genes play roles in survival in a hypoxic environment, production of a calcium-binding protein-inducing cytokine, and regulation of transcription in a toxin-antitoxin system. The gene expressions of IL-1β, IL-6, and IL-8, caspase-4, and NLRP3, but not of caspase-3 and caspase-9, were augmented in Mtb bacilli-infected fibroblasts. Taken together, our study suggests that Mtb bacilli attempt to survive in lung fibroblasts and that pyroptosis of the host fibroblasts activates the immune system against the infection.
Importance: The role of "non-classical immune cells," that is, fibroblasts, epithelial cells, adipocytes, etc., except for the "classical immune cells," that is, macrophages and lymphoid cells, is not well known in the infection of Mtb bacilli. We have previously found that live, but not dead, Mtb bacilli induce cell death in human lung fibroblasts, except in human macrophages and monocytes. The present study reveals that fibroblasts ingest Mtb bacilli the same as macrophages and that in vivo Mtb bacilli within fibroblasts attempt to survive in the host cells, and pyroptosis, including the production of inflammatory cytokines, is induced in the Mtb-infected fibroblasts. Our results suggest that pyroptosis of the host fibroblasts activates the immune system against the infection.
Keywords: Mycobacterium tuberculosis; RNA-Seq; caspase; cytokine; fibroblasts; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Takii T, Abe C, Tamura A, Ramayah S, Belisle JT, Brennan PJ, Onozaki K. 2001. Interleukin-1 or tumor necrosis factor-alpha augmented the cytotoxic effect of mycobacteria on human fibroblasts: application to evaluation of pathogenesis of clinical isolates of Mycobacterium tuberculosis and M. avium complex. J Interferon Cytokine Res 21:187–196. doi: 10.1089/107999001750133258 - DOI - PubMed
-
- Tamura M, Tokuda M, Nagaoka S, Takada H. 1992. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (Porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun 60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992 - DOI - PMC - PubMed
-
- Rastogi N, Labrousse V, de Sousa JP. 1992. Mycobacterial growth and ultrastructure in mouse L-929 fibroblasts and bone marrow-derived macrophages: evidence that infected fibroblasts secrete mediators capable of modulating bacterial growth in macrophages. Curr Microbiol 25:203–213. doi: 10.1007/BF01570720 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

