Nuanced differences in adenylate cyclase toxin production, acylation, and secretion may contribute to the evolution of virulence in Bordetella species
- PMID: 40387377
- PMCID: PMC12153271
- DOI: 10.1128/mbio.01082-25
Nuanced differences in adenylate cyclase toxin production, acylation, and secretion may contribute to the evolution of virulence in Bordetella species
Abstract
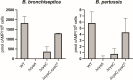
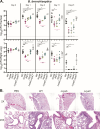
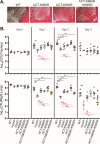
Bordetella pertussis, which causes the acute human disease whooping cough, evolved from Bordetella bronchiseptica, which causes long-term, chronic infections in a broad range of mammals. Both B. pertussis and B. bronchiseptica produce adenylate cyclase toxin (ACT), a bifunctional protein with adenylate cyclase-mediated cell intoxication and pore-forming activities. CyaC-mediated acylation of ACT is important for cell intoxication and required for pore-forming activity in vitro, but its role in vivo was unknown. Our comparative analysis showed that although ACT secreted by B. pertussis is fully acylated at residues K860 and K983, only a fraction of ACT secreted by B. bronchiseptica is modified. We showed that B. bronchiseptica produces more ACT than B. pertussis and is more efficient at ACT-dependent intoxication of macrophages in vitro than B. pertussis, but for both organisms, acylation of ACT greatly enhances intoxication. Acylation also enhances ACT secretion. Using a natural-host model, we determined that non-acylated ACT is functional during the initial stage of B. bronchiseptica infection, but not at later time points, and that acylation of K860, but not K983, is required for persistence in the lower respiratory tract. These data indicate a role for both acylated and non-acylated ACT during infection. Acylation of ACT was similarly not absolutely required for B. pertussis persistence in the murine lower respiratory tract. Overall, our data revealed nuanced differences in production, acylation, and secretion of ACT between B. pertussis and B. bronchiseptica that may correlate with their different virulence strategies.IMPORTANCEBordetella pertussis causes the acute disease whooping cough and survives only in the human respiratory tract, while Bordetella bronchiseptica causes long-term, chronic infections in a broad range of mammals and can also survive in extra-host environments. These bacteria produce a nearly identical set of virulence factors, including adenylate cyclase toxin (ACT), a protein that is modified by the addition of acyl chains. Acylation is required for ACT to cause hemolysis and for efficient intoxication of host cells in vitro. We found that ACT acylation is also important, but not absolutely required, during infection. We also discovered differences in ACT production, acylation, and secretion between B. bronchiseptica and B. pertussis that may contribute to the different virulence strategies of these species. This study highlights the advantage of conducting comparative analyses between closely related species to better understand the evolution of virulence.
Keywords: Bordetella; Bordetella bronchiseptica; Bordetella pertussis; acylation; adenylate cyclase toxin; bacterial respiratory infection; post-translational modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MTG, Churcher CM, Bentley SD, Mungall KL, et al. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 35:32–40. doi: 10.1038/ng1227 - DOI - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI043986/AI/NIAID NIH HHS/United States
- R01 AI153160-A1, R21 AI177818, R01 AI129541, R01 AI43986, R01 AI094991/National Institute of Allergy and Infectious Diseases
- R21 AI177818/AI/NIAID NIH HHS/United States
- R01 AI129541/AI/NIAID NIH HHS/United States
- R01 AI094991/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
