DNMT1-Induced Downregulation of CBX7 Inhibits ERK Phosphorylation and Promotes Pancreatic Ductal Adenocarcinoma Progression
- PMID: 40387566
- PMCID: PMC12087528
- DOI: 10.1096/fj.202402903R
DNMT1-Induced Downregulation of CBX7 Inhibits ERK Phosphorylation and Promotes Pancreatic Ductal Adenocarcinoma Progression
Abstract
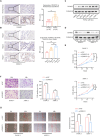
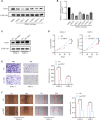
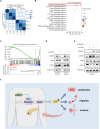
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive cancer types, characterized by an alarmingly low 5-year survival rate. DNA methylation has been implicated in the progression of various tumors, with DNA methyltransferase 1 (DNMT1) being the most extensively studied enzyme in this context. However, the expression patterns and underlying mechanisms of DNMT1 in PDAC remain poorly understood. The levels of DNMT1 and CBX7 in PDAC tissues and cells were determined by IHC and Western blot. ChIP and dual-luciferase reporter assays confirmed the interaction between DNMT1 and the CBX7 promoter. Cellular functions were evaluated through CCK-8, wound healing, and transwell assays. The expression of MAPK-related proteins was analyzed by Western blot. DNMT1 expression was upregulated in PDAC tissues and cell lines, whereas CBX7 expression was downregulated. Silencing DNMT1 inhibited cell proliferation, migration, and invasion in PDAC by modulating CBX7 expression. Moreover, DNMT1 methylates the CBX7 promoter region, leading to increased ERK phosphorylation, which subsequently drives tumorigenesis and metastasis in PDAC. DNMT1 promotes the malignant progression of PDAC through the CBX7/ERK pathway. Our study provides evidence for potential therapeutic targets for the comprehensive treatment of PDAC.
Keywords: CBX7; DNMT1; pancreatic ductal adenocarcinoma; promoter methylation.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Targeting of the G9a, DNMT1 and UHRF1 epigenetic complex as an effective strategy against pancreatic ductal adenocarcinoma.J Exp Clin Cancer Res. 2025 Jan 15;44(1):13. doi: 10.1186/s13046-024-03268-5. J Exp Clin Cancer Res. 2025. PMID: 39810240 Free PMC article.
-
Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4.J Exp Clin Cancer Res. 2018 Jul 3;37(1):130. doi: 10.1186/s13046-018-0807-2. J Exp Clin Cancer Res. 2018. PMID: 29970191 Free PMC article.
-
CBX7 suppresses cell proliferation, migration, and invasion through the inhibition of PTEN/Akt signaling in pancreatic cancer.Oncotarget. 2017 Jan 31;8(5):8010-8021. doi: 10.18632/oncotarget.14037. Oncotarget. 2017. PMID: 28030829 Free PMC article.
-
Downregulated expression of IL‑28RA is involved in the pathogenesis of pancreatic ductal adenocarcinoma.Int J Oncol. 2021 Aug;59(2):55. doi: 10.3892/ijo.2021.5235. Epub 2021 Jul 1. Int J Oncol. 2021. PMID: 34195850
-
DNMT1 as a therapeutic target in pancreatic cancer: mechanisms and clinical implications.Cell Oncol (Dordr). 2020 Oct;43(5):779-792. doi: 10.1007/s13402-020-00526-4. Epub 2020 Jun 5. Cell Oncol (Dordr). 2020. PMID: 32504382 Review.
References
-
- Bray F., Laversanne M., Sung H., et al., “Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries,” CA: A Cancer Journal for Clinicians 74, no. 3 (2024): 229–263. - PubMed
-
- Rahib L., Smith B. D., Aizenberg R., Rosenzweig A. B., Fleshman J. M., and Matrisian L. M., “Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States,” Cancer Research 74, no. 11 (2014): 2913–2921. - PubMed
-
- Rebecca L S., Angela N G., and Ahmedin J., “Cancer Statistics, 2024,” CA: A Cancer Journal for Clinicians 74, no. 1 (2024): 12–49. - PubMed
-
- Hu Z. I. and O'Reilly E. M., “Therapeutic Developments in Pancreatic Cancer,” Nature Reviews. Gastroenterology & Hepatology 21, no. 1 (2023): 7–24. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

