Immune-mediated adverse events following atezolizumab and bevacizumab in a multinational Latin American cohort of unresectable hepatocellular carcinoma
- PMID: 40387836
- PMCID: PMC12088043
- DOI: 10.18632/oncotarget.28721
Immune-mediated adverse events following atezolizumab and bevacizumab in a multinational Latin American cohort of unresectable hepatocellular carcinoma
Abstract
Aims: Latin America has been underrepresented in trials evaluating immunotherapy for hepatocellular carcinoma (HCC). We aimed to describe the incidence of immune-related adverse events (irAEs) and their impact on outcomes in a Latin American cohort.
Methods: A multicenter prospective study was conducted in Argentina, Brazil, Chile, and Colombia, including patients who received atezolizumab plus bevacizumab. A time-covarite proportional hazard analysis evaluated the effect of irAEs.
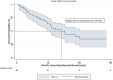
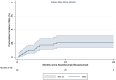
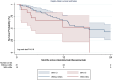
Results: 99 patients were included. The median treatment duration was 6 months, with a median survival of 17.0 months (95% CI: 12.6-19.8). The irAE incidence rate was 2.1 cases per 100 persons-months (cumulative incidence 18.1% (95% CI: 11.1-27.2%)). Median time to irAE was 2.3 months (range 1.4-4.8), most frequently hepatitis (n = 6), thyroiditis (n = 5), and 8/18 required steroids. Follow-up, treatment duration, and overall survival were similar regardless of the occurrence of irAEs (HR = 1.71, 95% CI: 0.76-3.86; P = 0.19). Baseline alpha-feto protein ≥400 ng/ml (HR: 2.9 (95% CI: 1.1-7.6)) was independently associated with irAE.
Conclusion: The incidence of irAEs in this cohort is lower than reported in controlled trials, withouut impact on survival outcomes. Education and early recognition are crucial to ensure that these events are identified and addressed.
Keywords: adverse events; immunology; immunotherapy; liver cancer; real-world.
Conflict of interest statement
The authors of this manuscript have the following conflicts of interest to disclose:
Leonardo Gomes da Fonseca, ORCID number 0000-0002-0216-3618. No direct conflicts of interest related to this research. Speaker honoraria, advisory board and grants from ROCHE, BAYER, Knight, Sirtex, MSD and Astra Zeneca.
Federico Piñero, ORCID number 0000-0002-9528-2279: No direct conflicts of interest related to this research. Speaker honoraria, advisory board and grants from ROCHE, BAYER, LKM Knight, RAFFO and Astra Zeneca. Has received a grant from the National Institute of Cancer (ID-190), and from the Argentine National Institute of Medical Innovation (PICT 2017-1986).
Margarita Anders, ORCID number 0000-0002-2238-5322: No direct conflicts of interest related to this research. Speaker honoraria, advisory board and grants from ROCHE, BAYER, Knight, RAFFO and Astra Zeneca.
Carla Bermudez: ORCID number 0000-0003-0082-9006. No direct conflicts of interest related to this research.
Ezequiel Demirdjian: No direct conflicts of interest related to this research.
Adriana Varón: No direct conflicts of interest related to this research.
Daniela Perez: No direct conflicts of interest related to this research.
Jorge Rodriguez: No direct conflicts of interest related to this research.
Oscar Beltrán: No direct conflicts of interest related to this research.
Ezequiel Ridruejo ORCID number 0000-0002-3321-0683. No direct conflicts of interest related to this research.
Alexandre Araujo: No direct conflict of interest related to this research.
Pablo Caballini: No direct conflicts of interest related to this research.
Juan Diego Torres Florez: No direct conflicts of interest related to this research.
Juan Ignacio Marín: No direct conflicts of interest related to this research.
Federico Orozco: No direct conflicts of interest related to this research.
Marina Villa: No direct conflicts of interest related to this research.
Jaime Poniachik: ORCID number 0000-0001-7958-3357. No direct conflicts of interest related to this research.
Sebastián Marciano: ORCID number 0000-0002-7983-1450. No direct conflicts of interest related to this research.
Fernando Bessone: ORCID number 0000-0002-8569-8123. No direct conflicts of interest related to this research.
Manuel Mendizabal: ORCID number 0000-0002-7026-9908. No direct conflicts of interest related to this research. Speaker honoraria, advisory board and grants from ROCHE, GADOR, MSD and Astra Zeneca.
Figures
References
-
- Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, et al.. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022; 76:681–93. 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

