Treatment Status for Generalized Pustular Psoriasis in Japanese Patients: A Retrospective Chart Review
- PMID: 40388057
- PMCID: PMC12126438
- DOI: 10.1007/s13555-025-01429-8
Treatment Status for Generalized Pustular Psoriasis in Japanese Patients: A Retrospective Chart Review
Abstract
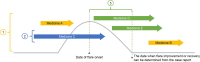
Introduction: Generalized pustular psoriasis (GPP) is a rare, severe, and chronic inflammatory skin disease characterized by widespread pustules that leads to unpredictable and potentially serious disease flares. Information regarding treatment status for GPP and treatment patterns for flares is important but limited as a result of the rarity of the condition. We conducted a 10-year, retrospective, longitudinal chart review of treatment patterns at GPP referral hospitals in Japan.
Methods: Eligible patients with GPP had at least 6 months of continuous observation data within 10 years after the date of protocol approval. Data were collected from patient records and annual patient reports. Patient characteristics and treatment details, including in relation to flare occurrence, were analyzed.
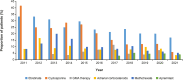
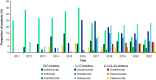
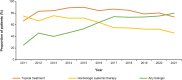
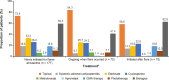
Results: The median age of patients (N = 205) was 53 years; 48.3% were female and most had mild or moderate GPP (66.8%). Patients commonly received nonbiologic systemic therapy (86.3%) and a similar proportion received biologics (79.5%); 95.1% received topical treatment and 22.4% received systemic adrenal corticosteroids. Use of nonbiologic systemic therapy decreased, and use of biologics increased, over the study period. During the observation period, the proportion of patients receiving biologic therapy increased after a flare (from 41.4% receiving biologics when flares occurred to 62.9% initiating a new biologic post flare).
Conclusion: In Japanese clinical practice, the evolution of treatment practices for GPP has seen an increased use of biologic therapies over time. Biologic use was common after flares; however, some flares occurred during biologic therapy, indicating a need for improved treatment options to maintain stable disease and prevent flares.
Keywords: Japan; Pustular psoriasis; Retrospective studies; Symptom flare-up; Therapeutics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Yukari Okubo declares receiving grants or contracts from Eisai, Maruho Pharmaceutical, and Torii Pharmaceutical, and consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen, JIMRO, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Novartis, Pfizer, Sanofi, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Ryuhei Okuyama declares receiving research grants, consulting fees, and/or speaker fees from AbbVie, Amgen, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Novartis, Pfizer, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Shinichi Imafuku has served as a consultant for, paid speaker for, accepted a research grant from, and/or participated in clinical trials sponsored by companies, including AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly, GSK, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Meiji Seika, Novartis, Pfizer, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, Toyo Seiyaku Kasei, and UCB Japan. Yayoi Tada declares receiving honoraria and/or grants from AbbVie, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Keiichi Yamanaka has served as a clinical trial investigator for AbbVie, Boehringer Ingelheim, Eli Lilly Japan, LEO Pharma, Janssen, Maruho Pharmaceutical, Merck Sharp & Dohme, Parexel, and UCB Japan; received research funding from AbbVie, Eisai, Eli Lilly Japan, Kyowa Kirin, Maruho Pharmaceutical, Sasaki Chemical, Sun Pharmaceutical Industries, Taiho Pharmaceutical, and Torii Pharmaceutical; and received speaker fees and Chair fees from AbbVie, Astellas Seiyaku, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eisai, Eli Lilly Japan, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Nippon Kayaku, Nippon Zoki, Novartis, Sato Seiyaku, Sanofi, Sun Pharmaceutical Industries, Taiho Pharmaceutical, and Torii Pharmaceutical. Kazumitsu Sugiura declares receiving research grants, consulting fees, and/or speaker fees from AbbVie, Amgen, Bristol Myers Squibb, Eisai, Eli Lilly Japan, Janssen, Kyowa Kirin, LEO Pharma Japan, Maruho Pharmaceutical, Nippon Boehringer Ingelheim, Novartis, Taiho Pharmaceutical, Sun Pharmaceutical Industries, Torii Pharmaceutical, and UCB Japan. Yukie Yamaguchi declares receiving research grants, consulting fees, and/or speaker fees from AbbVie, Amgen, Astellas, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Japan. Masahito Yasuda declares receiving consulting fees and/or speaker fees from Boehringer Ingelheim, Janssen, Taiho Pharmaceutical, and Novartis, and participating in clinical trials sponsored by Eli Lilly. Wataru Sakamoto and Morihisa Saitoh are employees of Nippon Boehringer Ingelheim. Akimichi Morita declares receiving research grants, consulting fees, and/or speaker fees from AbbVie, Boehringer Ingelheim, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho Pharmaceutical, Mitsubishi Tanabe, Nichi-Iko, Nippon Kayaku, Novartis, Sun Pharmaceutical Industries, Taiho Pharmaceutical, Torii Pharmaceutical, and Ushio. Ethical Approval: The study protocol was approved by the ethics review committee of each institute as detailed in the supplementary information of the previous publication [24]. The study was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients (or their proxy if the patient was a minor or unable to provide informed consent) at a visit to the study site before registration of the patient on the electronic data capture system. Patients who were unlikely to visit the hospital because of transfer to a different hospital, or patients who had died, were registered for electronic data capture but were then opted out of follow-up. Instructions for opt-out were posted at the hospital or on its website.
Figures
References
-
- Fujita H, Terui T, Hayama K, et al. Japanese guidelines for the management and treatment of generalized pustular psoriasis: the new pathogenesis and treatment of GPP. J Dermatol. 2018;45(11):1235–70. - PubMed
-
- Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult-onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53(6):676–84. - PubMed
-
- Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31(11):1792–9. - PubMed
LinkOut - more resources
Full Text Sources

