Effectiveness and Safety of Tezepelumab in a Diverse Population of US Patients with Severe Asthma: Initial Results of the PASSAGE Study
- PMID: 40388086
- PMCID: PMC12182456
- DOI: 10.1007/s12325-025-03231-6
Effectiveness and Safety of Tezepelumab in a Diverse Population of US Patients with Severe Asthma: Initial Results of the PASSAGE Study
Erratum in
-
Correction to: Effectiveness and Safety of Tezepelumab in a Diverse Population of US Patients with Severe Asthma: Initial Results of the PASSAGE Study.Adv Ther. 2025 Sep;42(9):4724-4725. doi: 10.1007/s12325-025-03316-2. Adv Ther. 2025. PMID: 40699278 Free PMC article. No abstract available.
Abstract
Introduction: Clinical trials for severe uncontrolled asthma (SUA) often underrepresent or exclude key patient populations. The phase 4 PASSAGE study assesses the effectiveness and safety of tezepelumab in a diverse, real-world US population of patients with SUA.
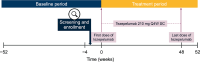
Methods: PASSAGE is an ongoing, multicenter, single-arm, open-label study of patients (≥ 12 years) with SUA. The study enrolled participants across asthma phenotypes, based on blood eosinophil counts (BECs) (≥/< 300 cells/µL) and perennial aeroallergen sensitization, and underrepresented subgroups (Black/African Americans, adolescents, participants with comorbid mild to moderate chronic obstructive pulmonary disease (COPD) and smokers [≥ 10 pack-years]). Participants receive tezepelumab 210 mg subcutaneously every 4 weeks for 52 weeks. This interim analysis assessed annualized asthma exacerbation rates (AAERs) in the 12-month baseline period (before starting tezepelumab) and the treatment period, and changes from baseline to week 24 in pre-bronchodilator (BD) forced expiratory volume in 1 second (FEV1), Asthma Control Questionnaire-6 (ACQ-6) score and health-related quality of life (HRQoL) outcomes.
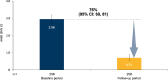
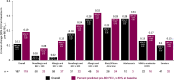
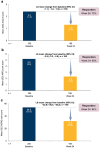
Results: Of 208 participants in this analysis, 41% had BEC ≥ 300 cells/µL, 56% had confirmed allergy, 17% were Black/African American, 5% were adolescents, 13% had comorbid COPD, and 23% were smokers. The AAER decreased by 76% (95% CI (confidence interval): 69, 81) from baseline to the treatment period; comparable reductions were observed across asthma phenotypes and underrepresented subgroups. The least squares mean change from baseline to week 24 in pre-BD FEV1 was 0.11 L (95% CI: 0.06, 0.17) overall and 0.19 L (95% CI: 0.12, 0.25) among participants with baseline pre-BD FEV1 ≤ 80% predicted. Clinically meaningful improvements from baseline to week 24 were observed for ACQ-6 score and HRQoL outcomes. No new safety signals were identified.
Conclusion: The PASSAGE study of US patients with SUA treated with tezepelumab demonstrates substantial reductions in AAERs across asthma phenotypes and underrepresented subgroups, with clinically meaningful improvements in lung function, asthma control and HRQoL.
Trial registration: ClinicalTrials.gov identifier, NCT05329194.
Keywords: Asthma; Biologic; Phenotype; Real-world; Severe asthma; Tezepelumab; Thymic stromal lymphopoietin; Underrepresented populations.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Njira L. Lugogo has received consultancy fees for participation in advisory boards from Amgen, AstraZeneca, Genentech, GSK, Novartis, Regeneron Pharmaceuticals, Sanofi and Teva Pharmaceuticals; has received honoraria for nonspeaker bureau presentations from AstraZeneca and GSK; has received speaker fees (own content) from NIOX; has received travel support from AstraZeneca; and has received research support to her institution from Amgen, AstraZeneca, Avillion, Genentech, Gossamer Bio, GSK, Regeneron Pharmaceuticals, Sanofi and Teva Pharmaceuticals. Praveen Akuthota has received consultancy fees and research support from Amgen, AstraZeneca, Connect Biopharma, GSK, Regeneron and Sanofi; has received research support from the American Partnership for Eosinophilic Disorders, the National Institutes of Health and Regeneron Pharmaceuticals; has received royalties from UpToDate; and has received fees for continuing medical education presentations from Advancing Knowledge in Healthcare, Medscape, MJH Life Sciences, PeerView, PRIME Continuing Medical Education, Projects in Knowledge, Rockpointe and Vindico Medical Education. Kaharu Sumino has received consultancy fees for participation in an advisory board from AstraZeneca. Sameer K. Mathur has received consultancy fees from AstraZeneca, GSK and Regeneron Pharmaceuticals, and has received fees for presentations from AstraZeneca and GSK. Autumn F. Burnette has received consultancy fees for participation in advisory boards and panel presentations from Amgen, AstraZeneca, GSK, Regeneron Pharmaceuticals and Sanofi, and has received speaker bureau fees from Amgen, AstraZeneca, Regeneron Pharmaceuticals and Sanofi. Andrew W. Lindsley and Jean-Pierre Llanos are employees of Amgen and own stock in Amgen. Claudio Marchese, Christopher S. Ambrose and Benjamin Emmanuel are employees of AstraZeneca and may own stock or stock options in AstraZeneca. Ethical Approval: The study is being conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation Good Clinical Practice guidelines and applicable regulatory requirements. Approvals from the Advarra Central Institutional Review Board (MD, USA and ON, Canada) and local independent ethics committees were obtained for all study sites (see supplementary material for a full list of ethics committees and Institutional Review Boards that covered the study sites). All participants provided written informed consent at the start of the study.
Figures

References
-
- Denton E, Price DB, Tran TN, et al. Cluster analysis of inflammatory biomarker expression in the International Severe Asthma Registry. J Allergy Clin Immunol Pract. 2021;9:2680–88 (e7). - PubMed
-
- Heaney LG, Perez de Llano L, Al-Ahmad M, et al. Eosinophilic and noneosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real-life severe asthma cohort. Chest. 2021;160:814–30. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. - PubMed
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention. Available at https://ginasthma.org/. Accessed Dec 12, 2024.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

