Macitentan and Tadalafil Combination Therapy in Patients with Pulmonary Arterial Hypertension and Cardiovascular Comorbidities: Real-World Evidence from OPUS and OrPHeUS
- PMID: 40388087
- PMCID: PMC12182455
- DOI: 10.1007/s12325-025-03180-0
Macitentan and Tadalafil Combination Therapy in Patients with Pulmonary Arterial Hypertension and Cardiovascular Comorbidities: Real-World Evidence from OPUS and OrPHeUS
Abstract
Introduction: Patients diagnosed with pulmonary arterial hypertension (PAH) often present with risk factors associated with cardiovascular disease, including diabetes mellitus (DM), hypertension (HTN), and obesity. The 2022 ESC/ERS pulmonary hypertension treatment guidelines recommend initial monotherapy with an endothelin receptor antagonist (ERA) or phosphodiesterase-5 inhibitor (PDE5i) for patients with PAH and cardiopulmonary comorbidities, with treatment escalation to be considered on an individual basis. Data on safety, tolerability, and effectiveness of combination therapy in these patients are lacking.
Methods: OPUS (prospective, observational drug registry) and OrPHeUS (retrospective, medical chart review) were multicenter US studies of patients newly initiating the ERA macitentan (2013-2020). Patients in the combined OPUS/OrPHeUS dataset with PAH receiving combination therapy with macitentan and the PDE5i tadalafil were identified. Descriptive analyses were performed for patients with ≥ 1 of DM/HTN/obesity and those without these comorbidities.
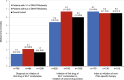
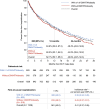
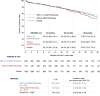
Results: In OPUS/OrPHeUS, 1336 patients with PAH received macitentan plus tadalafil during the observation period. Of these, 820 (61.4%) had ≥ 1 of DM/HTN/obesity and 516 (38.6%) had none of these comorbidities at or before enrollment. Median (Q1, Q3) exposure to macitentan and tadalafil combination therapy was similar at 13.7 (3.5, 28.0) and 14.8 (5.4, 27.4) months, respectively. For patients with ≥ 1 of DM/HTN/obesity versus those without, 1-year Kaplan-Meier estimates (95% confidence limits) for survival were 92.3% (89.9, 94.1) and 91.9% (88.8, 94.1), for patients free from hospitalization were 63.6% (59.6, 67.2) and 60.0% (55.1, 64.5), and for patients persisting on combination therapy were 66.5% (63.1, 69.8) and 68.5% (64.1, 72.4). Adverse events (AE; OPUS only) were reported in 78.4% and 80.0%, respectively, with no unexpected AEs observed. There was a trend towards higher AE incidence with increasing comorbidity number and in patients with cardiovascular comorbidities who were treatment-naïve.
Conclusion: Patients with PAH and ≥ 1 of diabetes mellitus, hypertension, or obesity treated with macitentan and tadalafil combination therapy had similar hospitalization, survival, and safety profiles as those without these comorbidities, though patients with comorbidities initiated on combination therapy and those with multiple comorbidities may require closer monitoring. These real-world data suggest that combination therapy may be considered for patients with PAH and cardiovascular comorbidities.
Trial registration: OPsumit® Users Registry (OPUS): NCT02126943; Opsumit® Historical Users cohort (OrPHeUS): NCT03197688; URL https://www.
Clinicaltrials: gov/.
Keywords: Cardiovascular comorbidities; Combination therapy; Hospitalization; Macitentan; Pulmonary arterial hypertension; Real-world data; Safety; Survival; Tadalafil.
Plain language summary
Patients with pulmonary arterial hypertension (PAH) often have other conditions affecting their heart or blood vessels, known as cardiovascular comorbidities. It is recommended that patients with PAH and cardiovascular comorbidities start with one PAH medication and later add another, if possible. However, there is limited information on the safety and effectiveness of combining two PAH medications in these patients. The OPUS and OrPHeUS studies collected information on patients with PAH treated in United States clinics between 2013 and 2020. We identified patients who were treated with two PAH medications, macitentan and tadalafil (referred to as combination therapy). We grouped them into patients with cardiovascular comorbidities (any medical history of diabetes mellitus, hypertension or obesity; 820 patients) and patients without these conditions (516 patients) and looked at how they did over time. Both groups were treated with combination therapy for approximately 14–15 months. After 1 year, outcomes were similar for patients with and without cardiovascular comorbidities: survival for both groups was 92%; 64% and 60% of patients remained hospitalization-free; and 67% and 69% remained on combination therapy. Side effects were consistent with those expected for these medications, with generally more side effects seen in patients with more comorbidities and in those new to PAH medications. Patients with PAH and cardiovascular comorbidities treated with macitentan and tadalafil combination therapy had similar results as those without cardiovascular comorbidities. This suggests that combination therapy may be used in select patients with PAH and cardiovascular comorbidities, although those who have more comorbidities or are new to PAH treatment may need closer observation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Nick H Kim has served as a Scientific Committee member for Johnson & Johnson; has received research grants / support from Johnson & Johnson, Bellerophon, Eiger, Gossamer Bio, Lung Biotechnology, SoniVie, and Altavant; has received consultant fees from Bayer, Merck, United Therapeutics, Pulnovo, and Polarean; and speaker fees from Johnson & Johnson and Bayer. Kelly M Chin has served as a Scientific Committee member for Johnson & Johnson; has received research grants / support from Johnson & Johnson, Altavant, Acceleron, United Therapeutics, Pfizer, Merck, Gossamer Bio; has received support for travel to meetings from Johnson & Johnson; and has received consultancy fees from Johnson & Johnson, Altavant, Acceleron, United Therapeutics, Gossamer Bio and Merck. Vallerie V McLaughlin served as a Scientific Committee member for Johnson & Johnson; received research grants from Aerovate, Altavant, Gossamer Bio, Johnson & Johnson, Merck, and SoniVie; and received consultant fees from Aerami, Aerovate, Altavant, Bayer, Caremark, Corvista, Gossamer Bio, Johnson & Johnson, L.L.C, Merck and United Therapeutics. Rose Ong is an employee of Actelion Pharmaceuticals Ltd, a Johnson & Johnson Company, holds stock/stock options in Johnson & Johnson and spouse is an employee of Roche. Gwen MacDonald and Nicolas Martin are employees of Actelion Pharmaceuticals Ltd, a Johnson & Johnson Company and hold stock/stock options in Johnson & Johnson. Assunta Senatore is an employee of Actelion Pharmaceuticals Ltd, a Johnson & Johnson Company. Richard Channick served as a Scientific Committee member for Johnson & Johnson; served on an advisory board for Johnson & Johnson and Bayer; received research grants / support from Johnson & Johnson and United therapeutics; received speaker fees from Johnson & Johnson, and Bayer; received consultancy fees from Johnson & Johnson, Bayer and Third pole. Ethical Approval: The OPUS and OrPHeUS studies were conducted according to Good Pharmacoepidemiology Practices and the 2008 Declaration of Helsinki ethical principles. Ethical approval was received from independent ethics committees/institutional review boards (IRB) of participating centers and overseen by an independent Scientific Committee (see Supplementary Appendix I for a full site list including local ethical review boards and Scientific Committee members); protocols were reviewed by the US FDA. Written informed consent obtained from all patients in OPUS, including for publication of anonymized patient data (informed consent was not required in OrPHeUS as an IRB waiver was obtained). The Informed Consent Form in OPUS included a confidentiality clause that all records and documents pertaining to the participation of patients in the OPUS registry would be held strictly confidential and their names would not be reported in any publications resulting from the OPUS registry. Separate master committees for OPUS and OrPHeUS provided overall ethical approval for the studies: IRB approvals were provided by WIRB and Quorum (now Advarra) (OPUS registry; WIRB approval number 2014‐0816, Quorum Review File number 29120/Advarra Pro00035124) and WCG‐IRB (OrPHeUS study; IRB numbers 2017‐8051 and 2017‐2348).
Figures
References
-
- Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United kingdom and ireland. Am J Resp Crit Care. 2012;186:790–6. 10.1164/rccm.201203-0383OC. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

