Serum sEV miRNAs as Biomarkers in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
- PMID: 40388105
- PMCID: PMC12367994
- DOI: 10.1007/s12035-025-04932-3
Serum sEV miRNAs as Biomarkers in Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease
Abstract
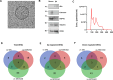
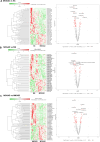
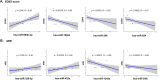
Myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) is a distinct CNS demyelinating disorder that differs from multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD). However, diagnosing MOGAD remains challenging due to the need to clinically exclude similar conditions and the variability in assay results. While liquid biomarkers have been extensively studied in MS and NMOSD, research on biomarkers for MOGAD remains limited. This study aims to investigate serum-derived small extracellular vesicle (sEV) miRNAs as potential diagnostic and prognostic biomarkers for MOGAD, distinguishing it from MS, NMOSD, and healthy controls. A comprehensive analysis of miRNAs in serum-derived sEVs was conducted to identify differentially expressed miRNAs among the groups. Correlations between miRNA profiles and clinical parameters, including the expanded disability status scale (EDSS) score and annualized relapse rate (ARR), were examined. The diagnostic potential of miRNAs was evaluated using the area under the curve (AUC) in the receiver operating characteristics (ROC) analyses. Serum samples were obtained from 47 patients (N = 11, MOGAD; N = 12, MS; and N = 12, NMOSD) and 12 healthy controls (HCs). We identified 77 dysregulated miRNAs in MOGAD patients, compared to HCs. Each three-miRNA panel demonstrated the highest AUC values for distinguishing MOGAD from HC (1.000), MOGAD from MS (0.939), and MOGAD from NMOSD (1.000). Additionally, hsa-miR-924 exhibited the strongest correlation with the EDSS score (ρ = -0.67, p < 0.001), while hsa-miR-548i showed the strongest correlation with ARR (ρ = -0.69, p < 0.001) in MOGAD. These miRNAs are involved in various pathways, including neuronal development, immune response, synaptic function, and chromatin remodeling, highlighting their potential roles in the pathophysiology of MOGAD. Serum sEV-derived miRNAs show strong potential as biomarkers for MOGAD, offering high diagnostic accuracy and correlations with clinical parameters. These findings pave the way for improved diagnostic and therapeutic strategies in MOGAD; however, further validation in larger cohorts is necessary.
Keywords: Annualized relapse rate; Biomarker; Expanded disability scale status; Extracellular vesicle; MiRNA; Multiple sclerosis; Myelin oligodendrocyte glycoprotein antibody-associated disease; Neuromyelitis optica spectrum disorder.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical Approval and Consent to Participate: A signed informed consent was received from all the participants. This study has been approved from the institutional review board of the Samsung Medical Center (IRB No. 2020–04 - 119). Consent for Publication: Signed informed consent was received from all the participants including consent for publication. Competing interests: JHM is funded by and has received research support from the National Research Foundation of Korea (MIST and KHIDI) and SMC Research and Development grant. She has lectured, consulted, and received honoraria from Bayer Healthcare, Merk, Biogen Idec, Sanofi, UCB, Samsung Bioepis, Mitsubishi Tanabe, Celltrion, Roche, Janssen, and AstraZeneca. All other authors declare that they have no competing interests.
Figures


Similar articles
-
Uncommon Non-MS Demyelinating Disorders of the Central Nervous System.Curr Neurol Neurosci Rep. 2025 Jul 1;25(1):45. doi: 10.1007/s11910-025-01432-8. Curr Neurol Neurosci Rep. 2025. PMID: 40591029 Review.
-
Reduced tolerogenic factor sCD83 in NMOSD and relapsing MOGAD: a potential new therapeutic pathway.Front Immunol. 2025 Jul 24;16:1620069. doi: 10.3389/fimmu.2025.1620069. eCollection 2025. Front Immunol. 2025. PMID: 40777014 Free PMC article.
-
Assessment of neuronal and glial serum biomarkers in myelin oligodendrocyte glycoprotein antibody-associated disease: the MULTIMOGAD study.J Neurol Neurosurg Psychiatry. 2025 Aug 14;96(9):884-892. doi: 10.1136/jnnp-2024-335137. J Neurol Neurosurg Psychiatry. 2025. PMID: 39939136
-
Optical coherence tomography angiography measurements in neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein antibody disease: A systematic review and meta-analysis.Mult Scler Relat Disord. 2024 Nov;91:105864. doi: 10.1016/j.msard.2024.105864. Epub 2024 Sep 2. Mult Scler Relat Disord. 2024. PMID: 39265270
-
A Differential Proteomic Analysis on Patient Sera with Neuromyelitis Spectrum Disorders (NMOSD) and Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD) Suggested Shared and Distinct Disease Mechanisms.J Proteome Res. 2025 Jul 4;24(7):3697-3714. doi: 10.1021/acs.jproteome.5c00382. Epub 2025 Jun 4. J Proteome Res. 2025. PMID: 40464632
References
-
- Marignier R, Hacohen Y, Cobo-Calvo A, Probstel AK, Aktas O, Alexopoulos H et al (2021) Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol 20(9):762–772 - PubMed
-
- Takai Y, Misu T, Kaneko K, Chihara N, Narikawa K, Tsuchida S et al (2020) Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain 143(5):1431–1446 - PubMed
-
- Jakimovski D, Bittner S, Zivadinov R, Morrow SA, Benedict RH, Zipp F et al (2024) Multiple sclerosis. Lancet 403(10422):183–202 - PubMed
-
- Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP et al (2023) Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. The Lancet Neurology 22(3):268–282 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

